cliniVec™

VectorBuilder's cliniVec™ program provides researchers with comprehensive solutions, facilitating the smooth transition from research and validation to pre-clinical studies. This seamless progression necessitates careful consideration of key factors such as the efficacy, safety, and manufacturability of cell and gene therapy drug products. The dedicated cliniVec™ design team at VectorBuilder focuses on optimizing vector designs specifically for pre-clinical and clinical studies.
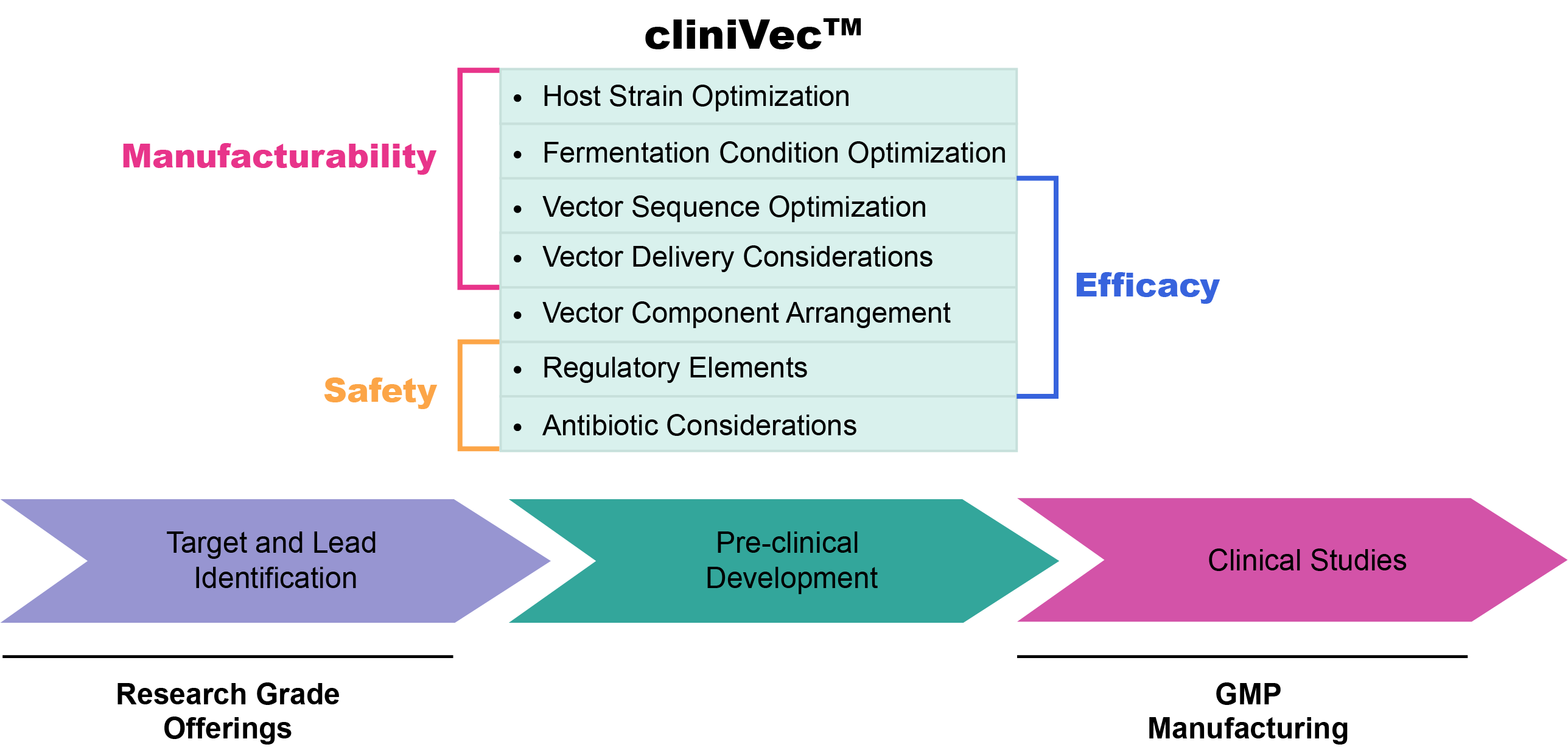
For personalized design support aligned with your project's unique needs, contact the VectorBuilder cliniVec™ design team, dedicated to helping you navigate the intricacies of pre-clinical vector design. It is imperative that researchers start optimizing their vector designs early in the pre-clinical process to conduct time-saving and cost-effective pre-clinical studies.
Vector Design Is Crucial for Efficacy
An optimal vector design is key for therapeutic efficacy and optimizing vector design is a multifaceted process that demands meticulous attention to various factors. Every vector component sequence should be thoughtfully designed and experimentally tested for better functionality. Promoter engineering plays a pivotal role in influencing gene expression levels, and careful manipulation of these regulatory regions can enhance the efficacy of a vector. Coding sequence optimization not only ensures the correct translation of the therapeutic gene but also facilitates its efficient expression. In cell and gene therapy development, this involves strategic measures such as optimizing GC content and CpG islands, addressing challenges like cryptic splice sites and premature PolyA signals, and ensuring optimal mRNA/IDR tertiary structure reduction. Collectively, these efforts contribute to efficient translation and improved therapeutic outcomes, reflecting the intricate nature of vector design optimization.
Besides the vector sequence itself, the arrangement of vector components is essential for seamless function and minimizing the risk of unintended interactions. In addition, considerations for efficient drug delivery, such as selecting appropriate vector systems and optimizing envelope proteins or capsids to target specific tissues, are equally important for successful clinical outcomes. The cliniVec™ design team has gathered a wealth of experience from designing thousands of custom vectors for research every year and offers comprehensive knowledge of the best methods for improving vector design for pre-clinical and clinical studies.
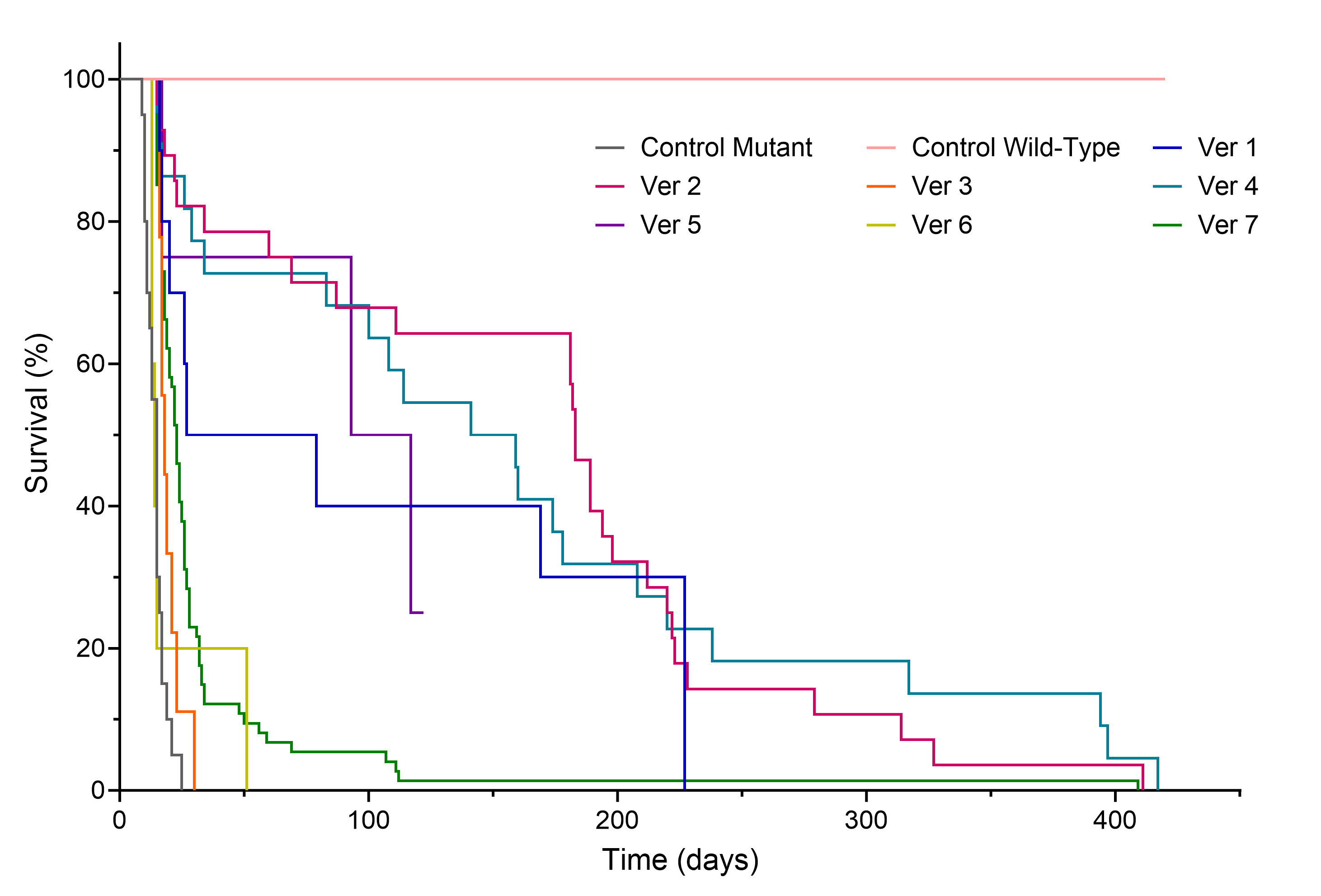
Figure 1. Efficacy study of promoter sequence optimization. Survival rates of transgenic knockout mice treated with gene replacement recombinant AAV9. This comparative analysis examines the efficacy of different sequence versions of the promoter driving GOI in the treatment of a genetic disorder. Control wild-type and non-treated control mutant mice were also observed.
Safety Considerations for Pre-clinical Vector Design
Ensuring the safety of cell and gene therapy vectors is paramount to their successful application in clinical settings. Drug developers must take into consideration a variety of areas including antibiotic selection, regulatory elements with viral origin, and toxicity and immunogenicity of vector components.
The choice of antibiotic selection marker in a pre-clinical vector is an especially important decision as many commonly used antibiotics are not compliant with clinical regulations. Therefore, it is suggested that Kanamycin be used starting from pre-clinical studies. In addition, the popular WPRE regulatory element raises safety concerns over the ORF it encodes, thus, an alternative like WPRE3 or a WPRE mutant is preferred.
Learn more about our miniaturaized miniVecTM plasmid with enhanced safety profile
Manufacturability Should Be Considered at Pre-clinical Stage
The manufacturability of gene therapy vectors involves multiple considerations to ensure scalability of upstream and downstream processes, reproducibility of yield and quality, and regulatory compliance. Yield optimization through vector backbone consideration, host strain optimization, and fermentation condition testing is essential for maximizing the quantity and quality of vector production. Optimizing both the specific yield and volume yield of pre-clinical vectors through these considerations results in higher quality DNA and more cost-effective manufacturing, respectively.
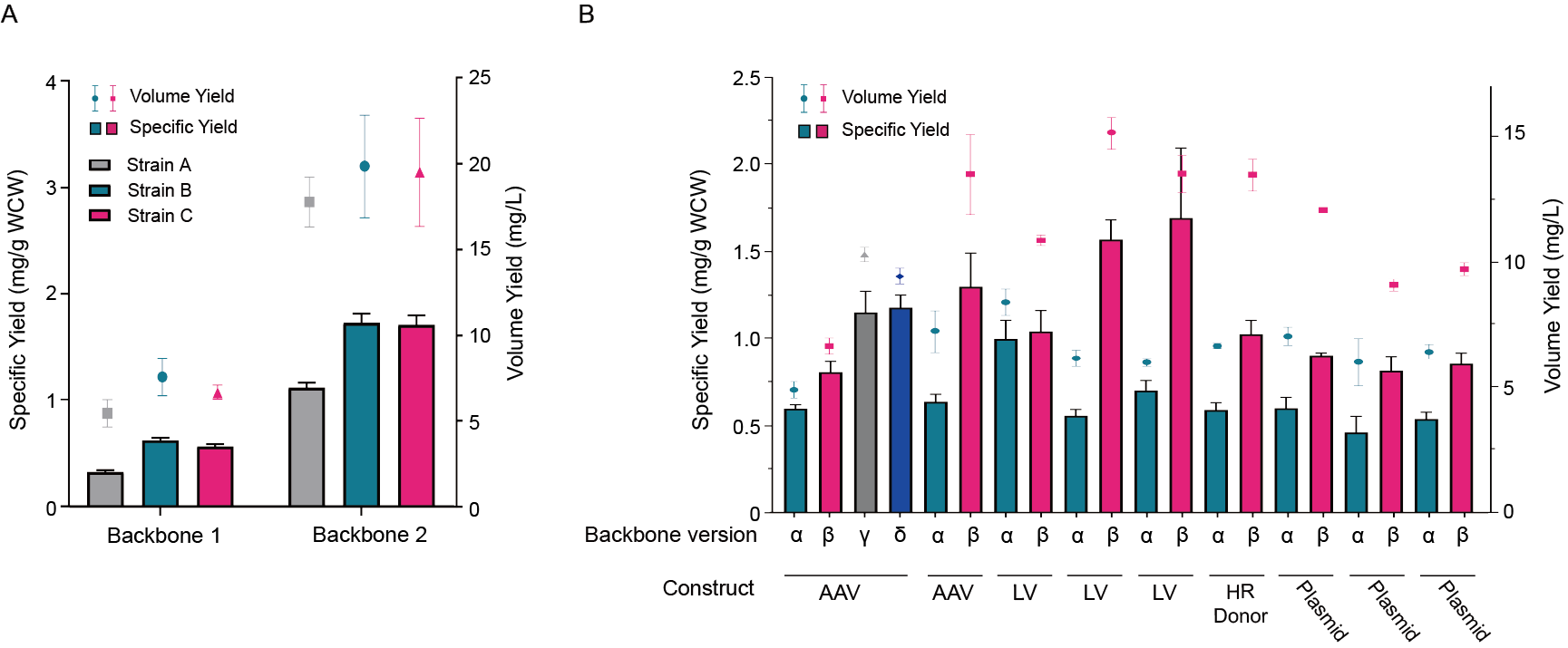
Figure 2. Yield optimization through backbone and host strain optimization. Vector constructs were cloned into different backbones and subjected to lab scale fermentation to test for specific (bar) and volumetric (dot) yields.
Experts in Sequence Screening
The cliniVec™ design team works closely with VectorBuilder’s expert in vitro and in vivo screening services to screen a wide variety of vector sequences for therapeutic applications. This involves a multifaceted approach including promoter and coding sequence screening, in addition to screening of vector delivery shells. We provide end-to-end screening services from library generation to NGS-sequencing and validation. Elevate the success of your vector development by partnering with the cliniVec™ design team for tailored screening solutions that meet the highest standards of precision and reliability.
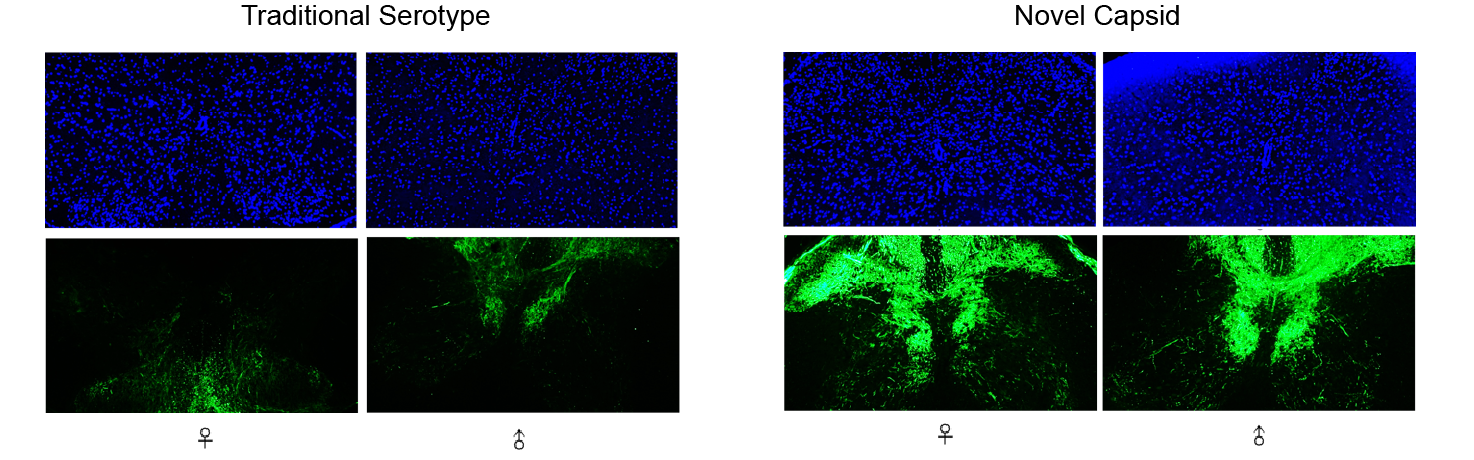
Figure 3. Optimization of vector delivery shells through AAV capsid evolution. Comparison of transgene delivery (CMV>EGFP) to mouse cervical spine from a traditional AAV serotype and a novel AAV serotype discovered through AAV capsid evolution and screening.



