Adenovirus Packaging
Recombinant adenoviral vectors are used for achieving high levels of transgene expression in a wide variety of mammalian cell types, in which the vector remains as episomal DNA without integration into the host genome. High transduction efficiency and high levels of short-term gene expression make adenoviral vector a preferred tool for in vivo gene delivery and they are often used in gene therapy and vaccination.
VectorBuilder has developed a series of proprietary technologies and reagents that have greatly improved recombinant adenovirus production protocols in terms of titer, purity, viability and consistency, especially for the adenoviral vector systems used in our vector cloning services. As a result, we have a growing base of highly satisfied customers who come back to us time after time for their adenoviral vector cloning and adenovirus packaging needs.
Types of adenovirus offered
- Human Ad5 adenovirus
- Chimeric Ad5/F35 adenovirus
- Gutless adenovirus
Service Details
Human Ad5 adenovirus
Chimeric Ad5/F35 adenovirus
Gutless adenovirus
Price and turnaround Price Match
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Pilot | Cell culture | >2x1010 IFU/ml | >1010 IFU/ml | 250 ul (10x25 ul) | $649 | 28-35 days |
| Medium | 1 ml (10x100 ul) | $1,099 | ||||
| Large | >2x1011 IFU/ml | >1011 IFU/ml | 1 ml (10x100 ul) | $1,699 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x1012 VP/ml | >1012 VP/ml | 500 ul (10x50 ul) | $2,099 | |
| Ultra-purified large | 1 ml (10x100 ul) | $2,499 |
IFU = Infectious units; VP = Virus particles
Deliverables
| For non-ultra-purified scales | For ultra-purified scales |
|---|---|
| Your custom Ad5 adenovirus | Your custom Ad5 adenovirus |
|
Free: control virus
|
Add-on purchase (optional): ultra-purified control virus
|
Control virus
The control adenovirus vector is designed to match the biological application of the custom virus and to be used for testing adenovirus transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control adenovirus (adenovirus overexpressing EGFP), and if the custom virus expresses an shRNA against a gene, then the control virus provided will express a scramble shRNA. Detailed information for the various control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| Adenovirus gene expression system | pAV[Exp]-CMV>EGFP | VB010000-9299hac |
| Adenovirus U6-based shRNA knockdown system | pAV[shRNA]-EGFP-U6>Scramble_shRNA | VB010000-0020ytx |
| Adenovirus miR30-based shRNA knockdown system | pAV[miR30]-CMV>EGFP:Scramble_miR30-shRNA | VB010000-9350dnk |
| Adenovirus CRISPR system | pAV[Exp]-CMV>EGFP | VB010000-9299hac |
Shipping and storage
Our non-ultra-purified adenovirus is stored in HBSS buffer and our ultra-purified adenovirus is stored in GTS buffer. Our adenovirus is shipped on dry ice. Upon receiving, adenovirus should be stored at -80°C for long term (stable for at least 1 year), or -20°C for short term, e.g., 2-3 weeks. Although adenovirus is more stable than many other virus (e.g. lentivirus) and can be frozen and thawed several times with minimal loss of virus activity, it is best to avoid repeated freeze-thaw cycles in practice.
Price and turnaround Price Match
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Pilot | Cell culture | >2x1010 IFU/ml | >1010 IFU/ml | 250 ul (10x25 ul) | $1,099 | 35-42 days |
| Medium | 1 ml (10x100 ul) | $1,699 | ||||
| Large | >2x1011 IFU/ml | >1011 IFU/ml | 1 ml (10x100 ul) | $2,599 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x1012 VP/ml | >1012 VP/ml | 500 ul (10x50 ul) | $3,199 | |
| Ultra-purified large | 1 ml (10x100 ul) | $3,799 |
IFU = Infectious units; VP = Virus particles
Deliverables
| For non-ultra-purified scales | For ultra-purified scales |
|---|---|
| Your custom chimeric Ad5/F35 adenovirus | Your custom chimeric Ad5/F35 adenovirus |
|
Free: control virus
|
Add-on purchase (optional): ultra-purified control virus
|
Control virus
The control Ad5/F35 adenovirus vector is designed to match the biological application of the custom virus and to be used for testing Ad5/F35 adenovirus transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control Ad5/F35 adenovirus (Ad5/F35 adenovirus overexpressing EGFP). Detailed information for the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| Ad5/F35 adenovirus gene expression system | pAd5/F35[Exp ]-CMV>EGFP | VB010000-9301bcw |
Shipping and storage
Our non-ultra-purified adenovirus is stored in HBSS buffer and our ultra-purified adenovirus is stored in GTS buffer. All our adenovirus are shipped on dry ice. Upon receiving, adenovirus should be stored at -80°C for long term (stable for at least 1 year), or -20°C for short term, e.g., 2-3 weeks. Although adenovirus is more stable than many other virus (e.g. lentivirus) and can be frozen and thawed several times with minimal loss of virus activity, it is best to avoid repeated freeze-thaw cycles in practice.
Price and turnaround Price Match
| Scale | Application | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|
| Ultra-purified medium | Cell culture & in vivo | >1011 VP/ml | 500 ul (10x50 ul) | $3,999 | 44-62 days |
| Ultra-purified large | Cell culture & in vivo | >1011 VP/ml | 1 ml (10x100 ul) | $4,999 | 44-62 days |
VP = Virus particles
Deliverables
| Your custom gutless adenovirus |
|
Add-on purchase (optional): ultra-purified control adenovirus
|
Control virus
The control gutless adenovirus is designed to match the biological application of the custom virus and to be used for testing gutless adenovirus transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control gutless adenovirus (gutless adenovirus overexpressing EGFP). Detailed information on the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| Gutless adenovirus gene expression system | pGLAd[Exp ]-CMV>EGFP | VB010000-9400ggg |
Shipping and storage
Our ultra-purified adenovirus is stored in GTS buffer. All our adenovirus are shipped on dry ice. Upon receiving, adenovirus should be stored at -80°C for long term (stable for at least 1 year), or -20°C for short term, e.g., 2-3 weeks. Although adenovirus is more stable than many other virus (e.g. lentivirus) and can be frozen and thawed several times with minimal loss of virus activity, it is best to avoid repeated freeze-thaw cycles in practice.
Technical Information
Human Ad5 adenovirus
Chimeric Ad5/F35 adenovirus
Gutless adenovirus
Adenovirus production and quality control (QC)
For our adenovirus manufacturing, the adenoviral vector carrying the gene of interest (GOI) is first linearized by restriction digestion with PacI. The linearized vector DNA is transfected into packaging cells expressing the adenovirus gene E1 to produce recombinant adenovirus, and adenoviral particles are released into tissue culture medium where they are collected. For ultra-purified adenovirus (in vivo grade), viral particles are further purified and concentrated by cesium chloride (CsCl) gradient ultracentrifugation. We measure the titer using an immunocytochemistry-based approach to detect the adenovirus-specific hexon protein. For ultra-purified adenovirus, we measure the optical density (using OD260) of the viral particles to estimate the titer.
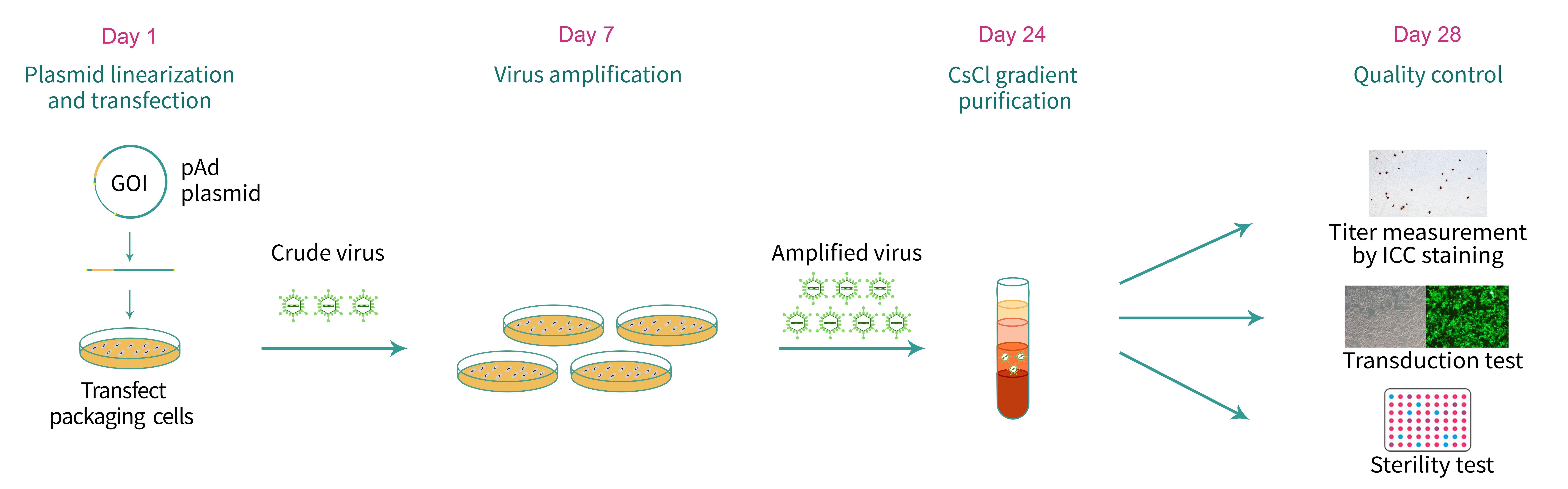
For each adenovirus produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the adenoviral vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. Additionally, for ultra-purified adenovirus, we routinely perform endotoxin assay to check the endotoxin level. To include the endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
Chimeric Ad5/F35 adenovirus production and quality control (QC)
For our chimeric Ad5/F35 adenovirus manufacturing (Figure 1), the Ad5/F35 adenoviral vector carrying the gene of interest (GOI) is first linearized by restriction digestion with PacI. The linearized vector DNA is transfected into packaging cells expressing the adenovirus gene E1 to produce recombinant adenovirus, and adenoviral particles are released into tissue culture medium where they are collected. For ultra-purified Ad5/F35 adenovirus (in vivo grade), viral particles are further purified and concentrated by cesium chloride (CsCl) gradient ultracentrifugation. We measure the titer using an immunocytochemistry-based approach to detect the adenovirus-specific hexon protein. For ultra-purified Ad5/F35 adenovirus, we measure the optical density (using OD260) of the viral particles to estimate the titer.
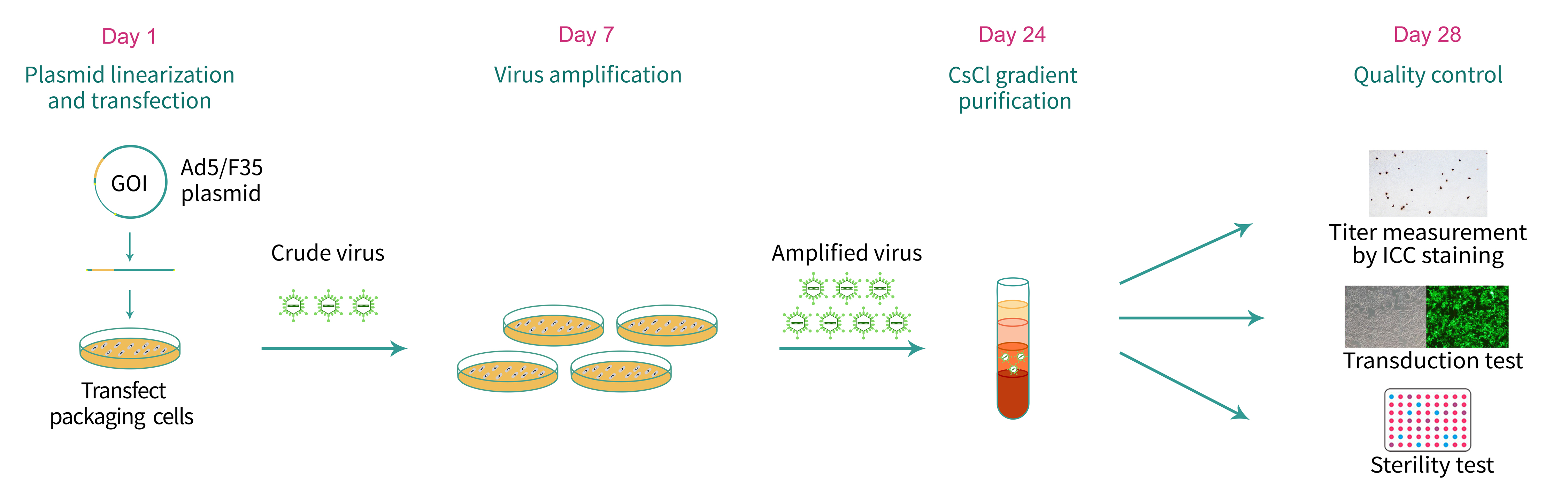
Figure 1. Typical workflow of chimeric Ad5/F35 adenovirus packaging.
For each adenovirus produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the Ad5/F35 adenoviral vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. Additionally, for ultra-purified adenovirus, we routinely perform endotoxin assay to check the endotoxin level. To include the endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
Ad5 adenovirus vs. chimeric Ad5/F35 adenovirus
While human adenovirus serotype 5 (Ad5) vectors are most widely used for adenovirus-based applications, a major limitation associated with such vectors is their dependence on the coxsackie and adenovirus receptor (CAR) for infecting target cells. Host cells that completely lack or have insufficient expression of CAR cannot be efficiently transduced with Ad5 vectors. Ad5/F35 vectors help overcome this limitation by expressing a chimeric fiber protein comprised of the knob and shaft derived from adenovirus serotype 35 (Ad35) and the fiber tail derived from Ad5. This enables Ad5/F35 adenoviral vectors to readily transduce CAR-negative cells or cells with low levels of CAR-expression in addition to CAR-positive cells with high levels of CAR expression, by exploiting the ability of the Ad35 fiber protein to attach to target cells via the non-CAR receptor, CD46. The chimeric design of Ad5/F35 adenovirus has been highly instrumental in expanding the tropism of adenoviral vectors to cell lines such as hematopoietic cells, primitive stem cells, vascular smooth muscle cells and CAR-negative tumor cells which otherwise exhibit poor transduction efficiency with conventional Ad5 vectors.
Experimental validation
We have developed a number of proprietary techniques to optimize our Ad5/F35 packaging protocol. Our optimized Ad5/F35 adenovirus has been validated to exhibit much higher transduction efficiencies in cells with insufficient CAR expression (Figure 2) than compared to conventional Ad5 adenovirus.
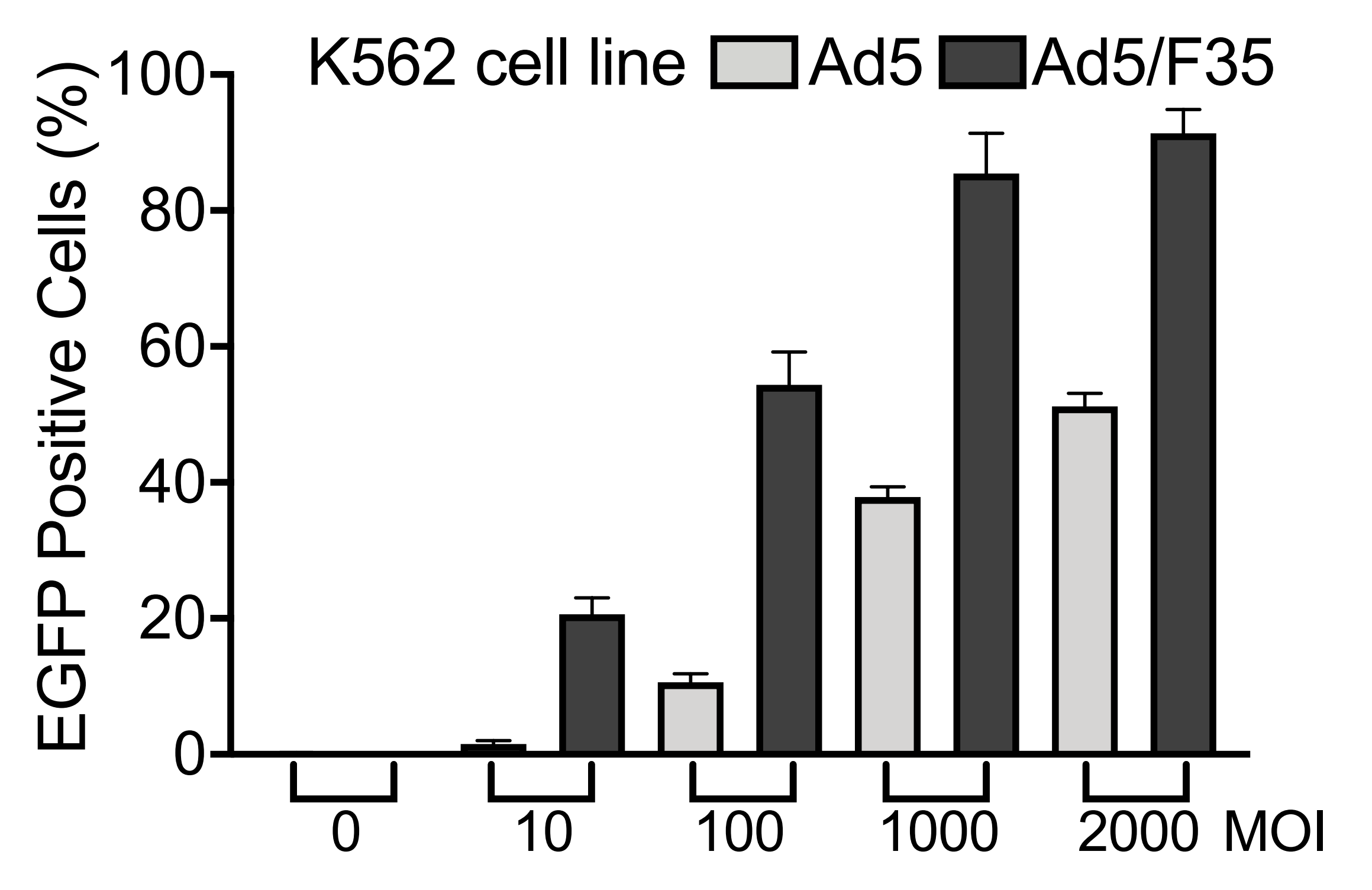
Figure 2. K562 cells were transduced with EGFP expressing Ad5 or Ad5/F35 adenovirus at increasing MOI and EGFP positive cells were analyzed by flow cytometry at 48 hours post-transduction. K562 cells exhibit low levels of CAR expression as shown by previous studies.
Gutless adenovirus production and quality control (QC)
For our gutless adenovirus manufacturing (Figure 1), an expression cassette containing the user-selected gene of interest (GOI) driven by a promoter is first cloned into our gutless adenovirus vector in between the two ITRs. Additionally, our vector incorporates stuffer sequences of appropriate lengths for maintaining a final size of above 28 kb between the two inverted terminal repeats (ITRs), which is necessary to facilitate the efficient packaging of virions. The region of the vector flanked by the two ITRs is then released from the plasmid by restriction digestion. The released fragment is transfected into packaging cells which are subsequently infected with the helper virus to generate recombinant adenoviral particles. Our gutless adenoviral vector system utilizes a helper virus with the packaging signal flanked by two LoxP sites along with packaging cells which stably express Cre recombinase to facilitate Cre-mediated excision of the helper virus packaging signal. This prevents the helper virus genome from being packaged into viral particles along with gutless adenoviral genome. The adenoviral particles are released into tissue culture medium where they are collected.
For ultra-purified gutless adenovirus (in vivo grade), viral particles are further purified and concentrated by cesium chloride (CsCl) gradient ultracentrifugation. We measure the optical density (using OD260) of the viral particles to estimate the titer.
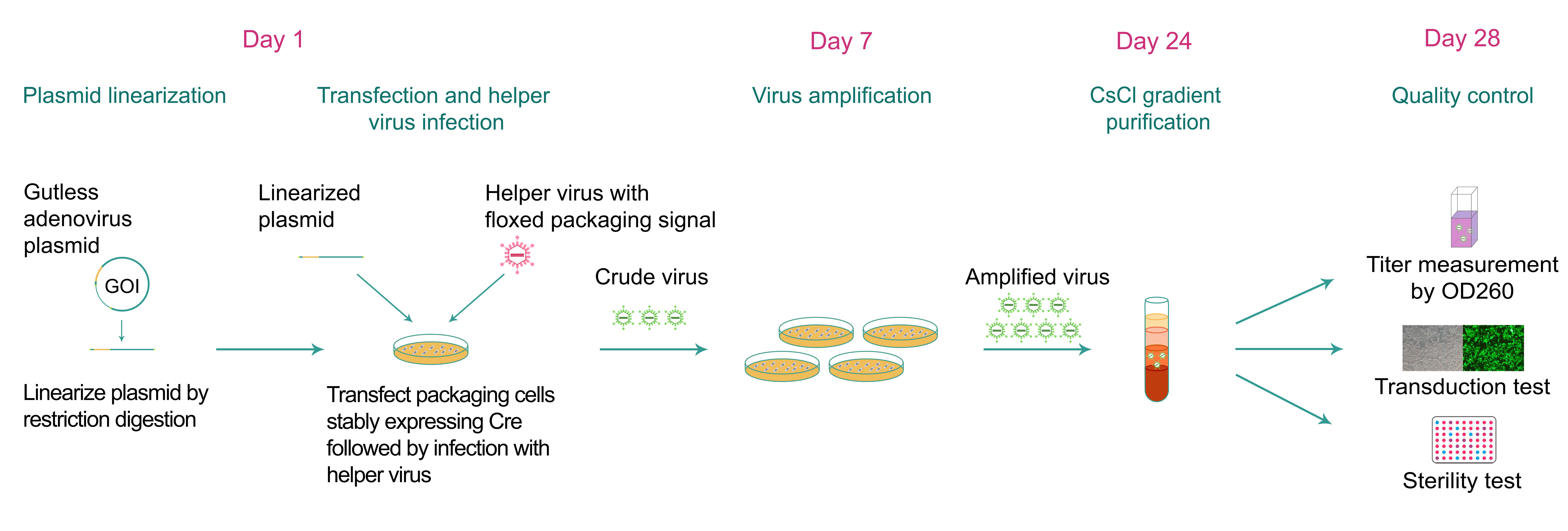
Figure 1. Typical workflow of gutless adenovirus packaging.
For each adenovirus produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the gutless adenoviral vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. Additionally, for ultra-purified adenovirus, we routinely perform endotoxin assay to check the endotoxin level. To include the endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
Advantages of gutless adenovirus over Ad5 and Ad/F35 adenovirus
Gutless adenoviral vectors (a.k.a. helper-dependent adenoviral vectors) are the latest generation of adenoviral vectors which offer several advantages over conventional Ad5 or chimeric Ad5/F35 adenoviral vectors. The absence of nearly all viral sequences on these vectors except cis-acting elements essential for viral replication and packaging enables gutless adenoviral vectors to exhibit minimal immunogenicity and prolonged transgene expression when used in vivo. Therefore, they have a significantly improved safety profile compared to Ad5 or Ad5/F35 which makes them highly attractive candidates for gene therapy applications. Additionally, the lack of viral coding sequences allows them to have an increased cargo carrying capacity of up to 33 kb, making them highly suitable for the expression of long or multiple transgenes. The table below summarizes the differences between different types of adenoviral vectors to help you select the right vector for your experiment:
| Human Ad5 adenovirus | Ad5/F35 adenovirus | Ad5 gutless adenovirus | |
|---|---|---|---|
| Structure | Derived from human Ad5 adenovirus with the portion of the genome in between the ITRs lacking the E1A, E1B and E3 genes. | Expresses a chimeric fiber protein comprised of the knob and shaft derived from Ad35 and the fiber tail derived from Ad5. Additionally, the portion of the genome between the two ITRs lack the E1A, E1B and E3 genes. | Derived from human Ad5 and characterized by the absence of all viral sequences except cis-acting elements essential for viral replication and packaging. |
| Cargo capacity | 7.5 kb | 8.2 kb | 33 kb |
| Tropism | Dividing and non-dividing cells with low transduction efficiency in cells lacking or having insufficient CAR expression. | Dividing and non-dividing cells with high transduction efficiency in both CAR positive and CAR negative cells. | Dividing and non-dividing cells with low transduction efficiency in cells lacking or having insufficient CAR expression |
| Immune response in vivo | High | High | Low |
| Helper virus needed | No | No | Yes |
Experimental validation
We have developed a number of proprietary techniques to optimize our gutless adenovirus packaging protocol. Our optimized gutless adenovirus has been validated to exhibit high transduction efficiency in mammalian cell lines.
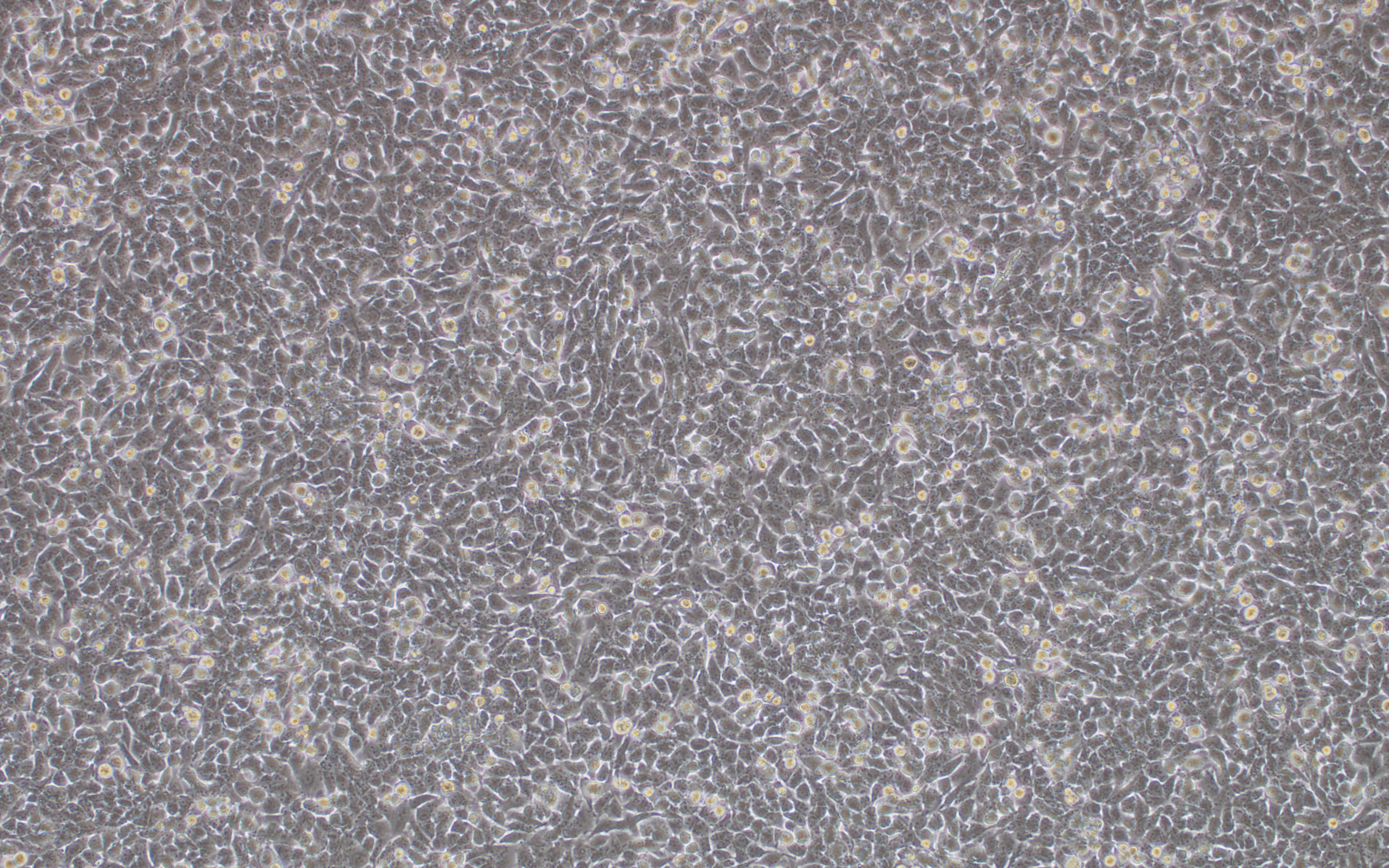
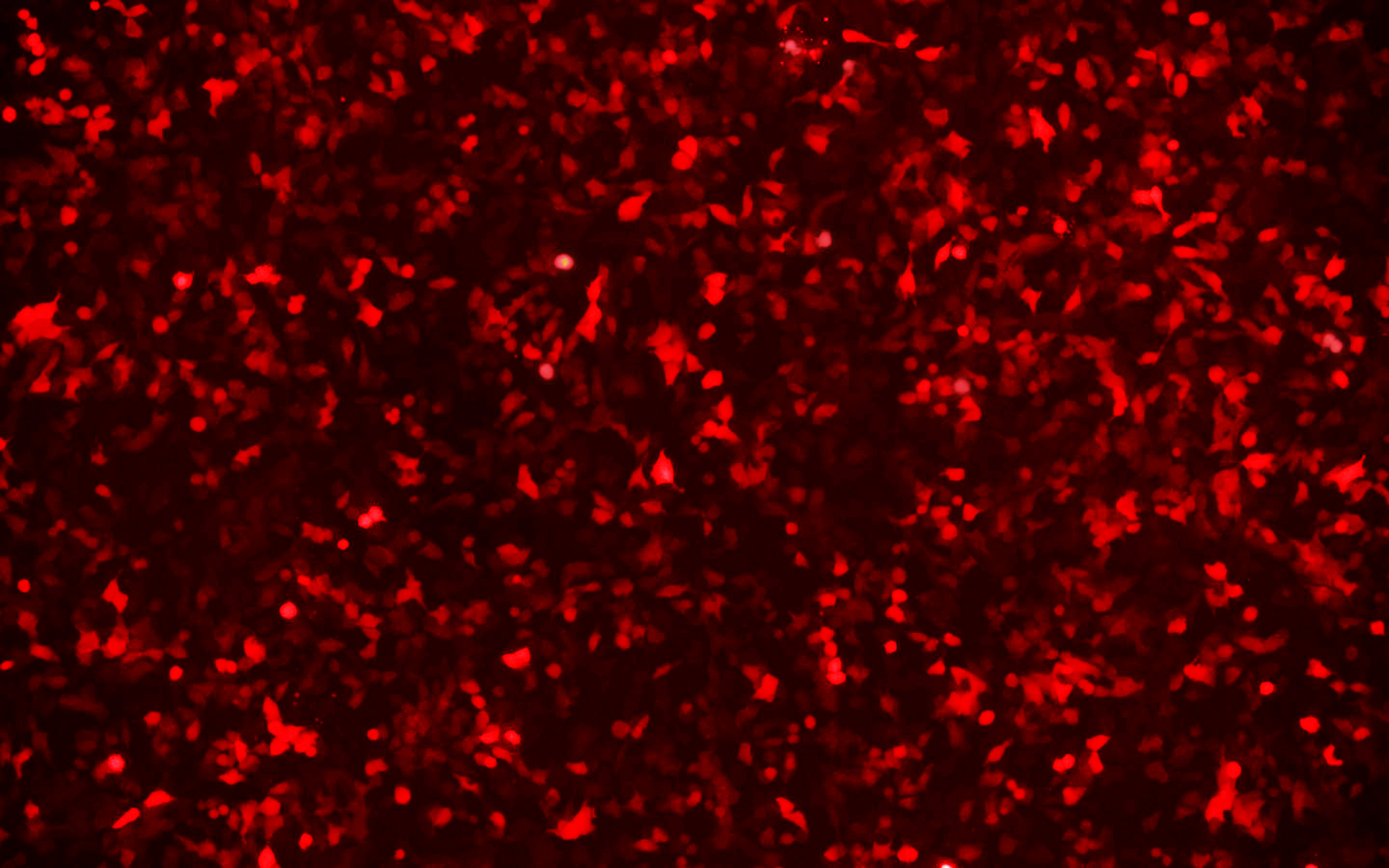
Figure 2. HeLa cells were transduced with mcherry expressing gutless adenovirus at an MOI of 5000. Magnification: 100x. Left: bright field. Right: mcherry.
How to Order
Customer-supplied vectors
If customer-supplied lentiviral plasmids are used in packaging, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
Resources
Documents
User Instructions Material Safety Data Sheet (MSDS) Certificate of Analysis (COA) FlyersFAQ
| Lentivirus | MMLV | Adenovirus | AAV | |
|---|---|---|---|---|
| Tropism | Broad | Broad | Ineffective for some cells | Depending on viral serotype |
| Can infect non-dividing cells? | Yes | No | Yes | Yes |
| Stable integration or transient | Stable integration | Stable integration | Transient, episomal | Transient, episomal |
| Maximum titer | High | Moderate | High | Very high |
| Promoter customization | Yes | No | Yes | Yes |
| Primary use | Cell culture and in vivo | Cell culture and in vivo | In vivo | In vivo |
| Immune response in vivo | Low | Low | High | Very low |
For adenovirus, we measure the functional titer. After transducing serially-diluted adenovirus into HEK293 cells, we use an immunocytochemistry-based approach to count the number of cells being successfully transduced via the detection of adenovirus-specific hexon protein, and each immunostained cell is considered as one infectious unit. Cells are infected at very low multiplicity of infection (MOI) to ensure that most transduced cells are each infected by a single viral particle. This assay shows good correlation with conventional plaque assay. For ultra-purified adenovirus, we directly measure the optical density (using OD260) of the viral particles to estimate titer, because there is a tight correlation between the optical density of ultra-purified adenovirus and functional titer. Adenovirus has very good stability. In our preparation, the viral particles are essentially all alive and can remain functional at room temperature for many days.
We guarantee the minimum titer when the region of the vector being packaged into virus (from 5’ ITR to 3’ ITR) is below the adenovirus cargo limit (38.7 kb). For sizes above this, the adenoviral genome may be unstable, and rearrangement may occur during packaging. Additionally, we are not able to guarantee titer for the following vectors:
- Vectors containing sequences that could adversely affect the packaging process such as toxic genes (e.g. proapoptotic genes), genes that compromise the integrity of packaging cells or virus (e.g. membrane proteins that cause cell aggregation), and sequences prone to rearrangements or secondary structures (e.g. repetitive or highly GC-rich sequences);
- Packaging of customer-supplied vector because we have no control over the quality of the vector and the compatibility of the vector with our packaging system.
The estimated turnaround is the time from production initiation to completion. It does not include waiting time for any customer-supplied materials (e.g. template DNA or viral vectors), QC of such materials, and transit time for shipping final deliverables to the customer.