Lentivirus Packaging
Recombinant lentivirus is the most commonly used viral vector for efficient gene delivery into mammalian cells. Unlike plasmid DNA vector which only allows transient and episomal expression of the foreign gene in the host cell, lentiviral vector can achieve permanent expression of the foreign gene through integration into the host cell genome. Lentivirus is also efficient for in vivo gene delivery.
VectorBuilder offers lentivirus packaging services with superior quality. We have developed a series of proprietary technologies and reagents that have greatly improved recombinant lentivirus production protocols in terms of titer, purity, viability and consistency, especially for the third-generation lentiviral vector systems used in our vector cloning services. As a result, we have a growing base of highly satisfied customers who come back to us time after time for their lentiviral vector cloning and lentivirus packaging needs. In addition to research-grade lentivirus, we also offer GMP-grade lentivirus manufacturing for clinical applications.
Types of lentivirus offered
- VSV-G pseudotyped third-generation lentivirus (this is the default virus type)
- VSV-G pseudotyped second-generation lentivirus
- Lentivirus pseudotyped with other envelope proteins as requested, such as coronavirus spike (S) proteins
- Bald lentivirus lacking viral envelope protein (this can be used as negative control in viral transduction)
- Integrase-deficient lentivirus (IDLV)
Service Details
Price and turnaround Price Match
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Mini | Cell culture | >2x108 TU/ml | >108 TU/ml | 100 ul (4x25 ul) | $199 |
6-12 days
|
| Pilot | >4x108 TU/ml | 250 ul (10x25 ul) | $449 | |||
| Medium | >3x108 TU/ml | 1 ml (10x100 ul) | $649 | |||
| Large | >2x109 TU/ml | >109 TU/ml | 1 ml (10x100 ul) | $1,099 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x109 TU/ml | >109 TU/ml | 500 ul (10x50 ul) | $1,399 | |
| Ultra-purified large | 1 ml (10x100 ul) | $1,699 |
Note:
1. The above table applies to VSV-G pseudotyped 2nd- and 3rd-generation lentivirus (integrase-deficient lentivirus (IDLV) included).
2. Click to view service details of lentivirus pseudotyped with coronavirus S protein.
3. TU = Transduction units (also known as infectious units).
Deliverables
| For non-ultra-purified scales | For ultra-purified scales |
|---|---|
| Your custom lentivirus | Your custom lentivirus |
|
Free: control virus
|
Add-on purchase (optional): ultra-purified control virus
|
|
Free: Polybrene (5 mg/ml, 200 ul) |
Free: Polybrene (5 mg/ml, 200 ul) |
Control virus
The control lentivirus is designed to match the biological application of the custom virus and to be used for testing lentiviral transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control lentivirus (lentivirus overexpressing EGFP), and if the custom virus expresses an shRNA against a gene, then the control virus provided will express a scramble shRNA. Detailed information on the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| Lentivirus gene expression system | pLV[Exp]-EGFP/Puro-EF1A>mCherry | VB010000-9298rtf |
| Lentivirus CRISPR system | pLV[Exp]-EGFP/Puro-EF1A>mCherry | VB010000-9298rtf |
| Lentivirus U6-based shRNA knockdown system | pLV[shRNA]-EGFP/Puro-U6>Scramble_shRNA | VB010000-0009mxc |
| Lentivirus miR30-based shRNA knockdown system | pLV[miR30]-EGFP/Puro-EF1A>mCherry:Scramble_miR30-shRNA | VB010000-9387zjj |
| Lentivirus IPTG-inducible shRNA knockdown system | pLV[shRNA]-LacI/Puro-U6/2xLacO>Scramble_shRNA | VB010000-9348prq |
Shipping and storage
Our lentivirus is stored in HBSS buffer and is shipped on dry ice. Upon receiving, it should be stored at -80°C for long term (stable for at least 6 months), or -20°C for use within one week. The shelf life for lentivirus is approximately one year. Please avoid repeated freeze-thaw cycles of lentivirus, as this can result in a large titer drop.
Technical Information
Lentivirus production and quality control (QC)
For our third-generation lentivirus packaging, transfer plasmid carrying the gene of interest (GOI) is co-transfected with our proprietary envelope plasmid encoding VSV-G and packaging plasmids encoding Gag/Pol and Rev into HEK293T packaging cells. After 48 hours of incubation, the supernatant is collected and centrifuged to remove cell debris and then filtered. Lentiviral particles are subsequently concentrated with PEG. For ultra-purified lentivirus (in vivo grade), viral particles are further purified by sucrose cushion ultracentrifugation and concentrated by ultrafiltration. We use the p24 ELISA method to measure lentivirus titer.
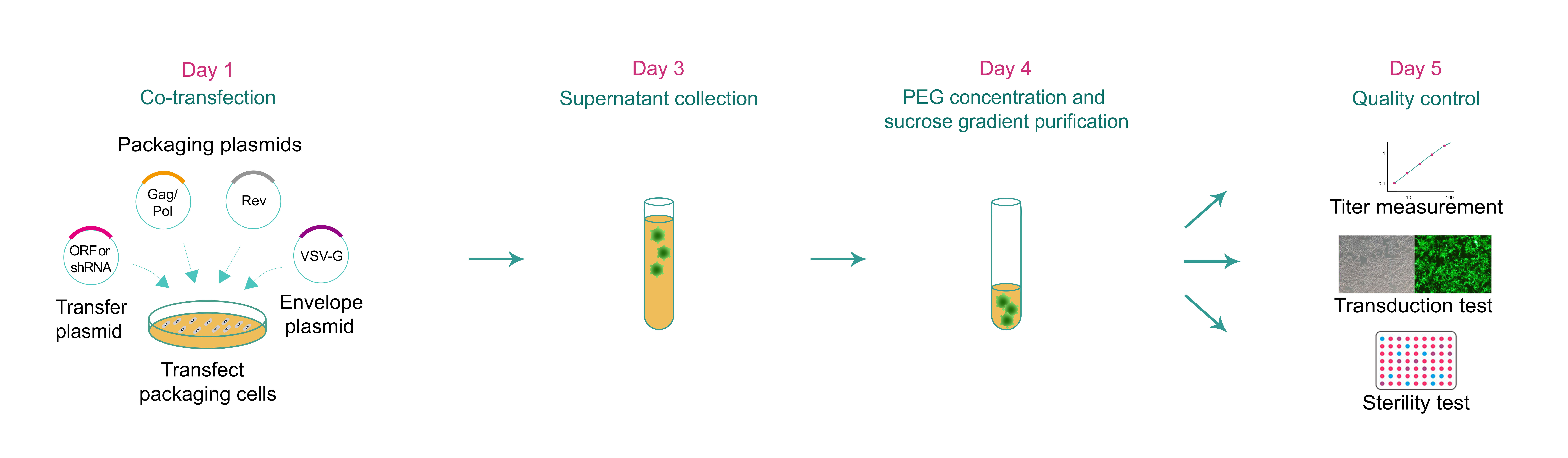
For each recombinant lentivirus produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the transfer vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. If the transfer vector encodes a drug-selectable marker, we would perform transduction test followed by corresponding drug selection. Additionally, for ultra-purified lentivirus, we routinely perform endotoxin assay to check the endotoxin level. To include the endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
How to Order
Customer-supplied vectors
If customer-supplied lentiviral plasmids are used in packaging, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
Resources
Documents
User Instructions Material Safety Data Sheet (MSDS) Certificate of Analysis (COA) Brochures & FlyersFAQ
| Lentivirus | MMLV | Adenovirus | AAV | |
|---|---|---|---|---|
| Tropism | Broad | Broad | Ineffective for some cells | Depending on viral serotype |
| Can infect non-dividing cells? | Yes | No | Yes | Yes |
| Stable integration or transient | Stable integration | Stable integration | Transient, episomal | Transient, episomal |
| Maximum titer | High | Moderate | High | Very high |
| Promoter customization | Yes | No | Yes | Yes |
| Primary use | Cell culture and in vivo | Cell culture and in vivo | In vivo | In vivo |
| Immune response in vivo | Low | Low | High | Very low |
We use the p24 ELISA for measuring lentivirus titer. This method employs a sandwich immunoassay to measure the levels of the HIV-1 p24 core protein in lentiviral supernatants. The lentivirus samples are first added to a microtiter plate, the wells of which are coated with an anti-HIV-1 p24 capture antibody, to bind the p24 in the lentivirus samples. This is followed by the addition of a biotinylated anti-p24 secondary antibody, which in turn binds to the p24 captured by the first antibody on the plate. A streptavidin-HRP conjugate is then added for binding the biotinylated anti-p24 antibody due to the interaction between streptavidin and biotin. A substrate solution is ultimately added to the samples which produces color upon interaction with HRP. The intensity of the colored product is proportional to the amount of p24 present in each lentivirus sample, which is measured by the use of a spectrophotometer and is then precisely quantified by comparing against a recombinant HIV-1 p24 standard curve. The p24 value is then correlated with the viral titer of the corresponding lentivirus sample.
Our titer guarantee applies to lentiviral vectors for which the region being packaged into virus (from Δ5’ LTR to ΔU3/3’ LTR) is below the lentivirus cargo limit (9.2 kb). For sizes above this, it may still be possible to package the vector into virus, but the titer may be reduced. Additionally, we are not able to guarantee titer for the following vectors:
- Vectors containing sequences that could adversely affect the packaging process such as toxic genes (e.g. proapoptotic genes), genes that compromise the integrity of packaging cells or virus (e.g. membrane proteins that cause cell aggregation), and sequences prone to rearrangements or secondary structures (e.g. repetitive or highly GC-rich sequences);
- Customer-supplied plasmids containing undisclosed sequences or atypical lentiviral functional elements (e.g. LTR) that may introduce uncertainties to packaging efficiency.
Our estimated turnaround is the time from production initiation to completion. It does not include waiting time for any customer-supplied materials (e.g. template DNA or viral vectors), QC of such materials, and transit time for shipping final deliverables to the customer.