Vaccinia Virus (VACV)
Recombinant VACV has several advantages including large carrying capacity (25-30 kb), wide tropism, and ability to replicate without host machinery. It has recently shown great promise in a broad range of clinical applications, including vaccine development and oncolytic therapy. VectorBuilder now offers both cloning and packaging services of VACV vectors, suitable for either in vitro or in vivo studies.
Types of VACV services offered
- VACV vector cloning in BACYAC backbone for both attenuated Western Reserve (WR) and Modified Vaccinia virus Ankara (MVA) strains
- VACV virus packaging
Service Details
VACV BACYAC vector cloning
VectorBuilder has developed a proprietary “BACYAC” backbone for cloning VACV vectors. This backbone combines key elements from both the bacterial artificial chromosome (BAC) and the yeast artificial chromosome (YAC). The BAC elements on the backbone allow the vector to behave like a BAC and be propagated in E. coli, whereas the YAC elements allow the vector to behave like a YAC and be propagated in the yeast Saccharomyces cerevisiae. Our BACYAC backbone thus provides users with the flexibility to grow and modify the vector in either E. coli or yeast. The BACYAC backbone is available with either LacZ or EGFP reporter that enables infected cells to be identified easily based on reporter expression.
The EGFP/LacZ reporter gene, BACYAC backbone, and vaccinia promoter are inserted into the vaccinia TK (thymidine kinase) gene between J2R sites, providing an attenuated recombinant vector. The MVA and attenuated WR strains can be used in basic research, preclinical, and clinical settings. We are also able to produce further attenuated viruses through directed mutations.
The basic design of our VACV BACYAC vector is illustrated in Figure 1 below.
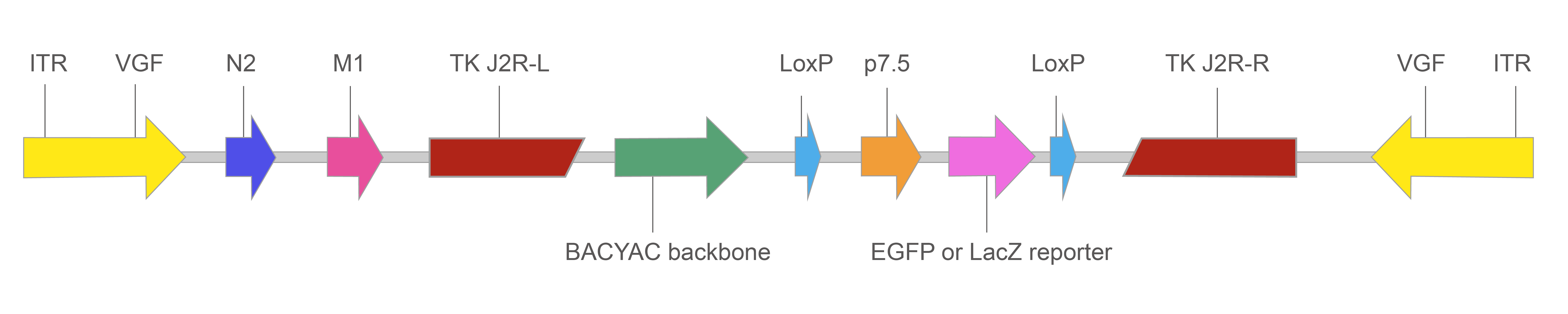
Figure 1. Map of recombinant VACV BACYAC vector with EGFP/LacZ reporter.
TK: Thymidine kinase gene, inactivated in the recombinant VACV to result in an attenuated virus.
p7.5: Vaccinia promoter. Because this virus replicates in the cytoplasm, a vaccinia promoter is required. p7.5 is commonly used for driving transgene expression because it is transcriptionally active in the early and late stages of the virus.
LoxP: Recombination site for Cre recombinase. When Cre is present, the region flanked by the two loxP sites will be excised.
ITR: Inverted terminal repeats flanking the vaccinia genome, containing the VGF (viral growth factor) gene.
N2: Gene expressed during early infection that inhibits host immune response.
M1: Gene that prevents apoptosis of the host cell, enhancing viral production.
EGFP or LacZ reporter: EGFP or LacZ driven by CMV promoter. It enables infected cells to be identified easily based on reporter expression.
BACYAC backbone: Bacterial artificial chromosome (BAC) and yeast artificial chromosome (YAC) backbone. It allows the vector to be selectively propagated in E. coli using chloramphenicol resistance gene and Saccharomyces cerevisiae using His3 auxotrophic selection marker.
VACV virus packaging
Price and turnaround Price Match
| Scale | Application | Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|
| Pilot |
Cell culture |
>108 PFU/ml | 250 ul (10x25 ul) | $399 | 21-28 days |
| Medium |
Cell culture |
>108 PFU/ml | 1 ml (10x100 ul) | $1,699 | 21-28 days |
| Ultra-purified medium | Cell culture & in vivo | >108 PFU/ml | 1 ml (10x100 ul) | $2,699 | 21-35 days |
Shipping and storage
Our VACV is stored in HBSS buffer and is shipped on dry ice. Upon receiving, it should be stored at -80°C long term (stable for at least 6 months), or -20°C for use within one week. The shelf life for VACV is approximately one year. Please avoid repeated freeze-thaw cycles of VACV, as this can result in a large titer drop.
Technical Information
VACV production and quality control (QC)
For virus packaging (Figure 2), a noninfectious BACYAC plasmid carrying the gene of interest (GOI) is transfected into packaging cells and infected with a helper non-replicating fowlpox virus. Because VACV is capable of infecting a broad range of host cells, multiple cell lines can be used to package the recombinant virus. At VectorBuilder different cell lines are used for VACV rescuing and amplification in preparation of virus stocks. Following VACV rescuing and amplification steps, plaque purification is used to establish a clonal virus stock which is then further amplified. Both extracellular enveloped virus (EEV) and intracellular mature virus (IMV) are harvested from the supernatant and cell lysate, respectively. EEV is PEG concentrated and combined with IMV. For ultra-purified VACV, viral particles are further purified by sucrose gradient purification.
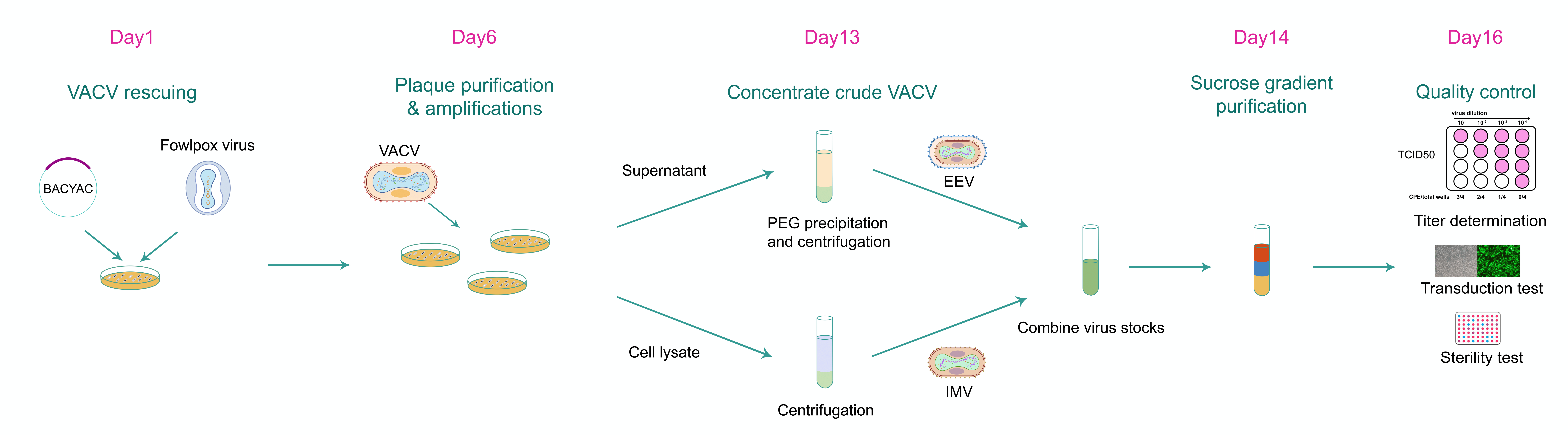
Figure 2. Typical workflow of VACV packaging from BACYAC vector.
For each recombinant VACV produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the VACV vector encodes a fluorescent protein or drug-selection marker, we would perform a transduction test with fluorescence detection or drug selection, respectively. Additionally, for ultra-purified VACV, we routinely perform endotoxin assays to check the endotoxin level. To include endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
Experimental validation
Our mature VACV has been fully validated and shown to be capable of transduction into BHK21 cell line (Figure 3).
Before transduction After transduction
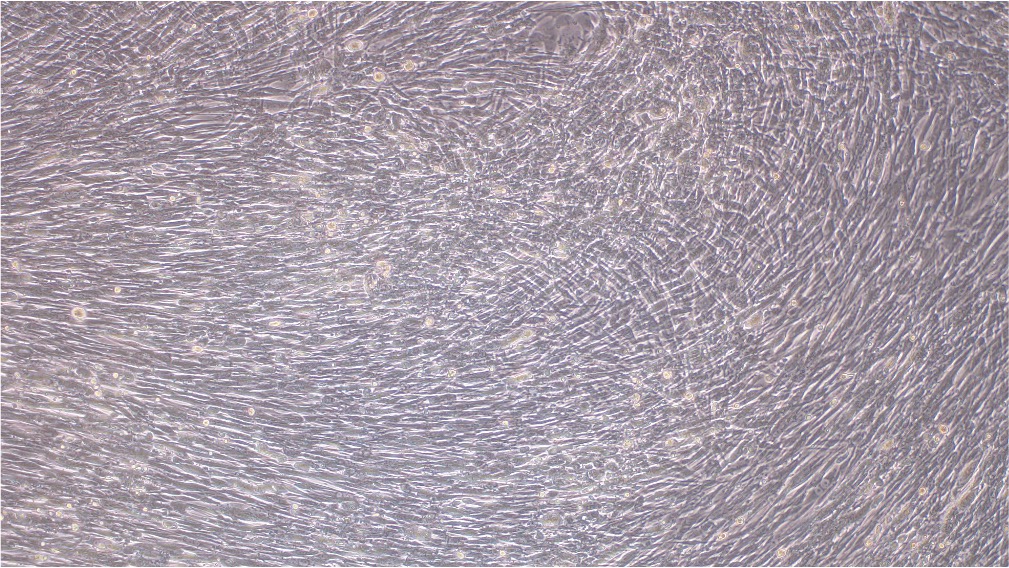

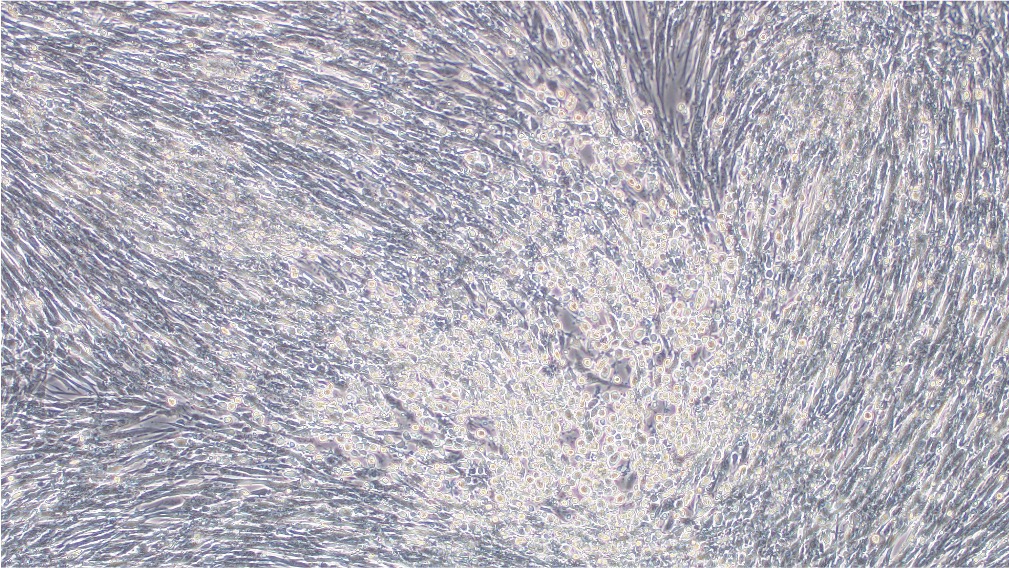
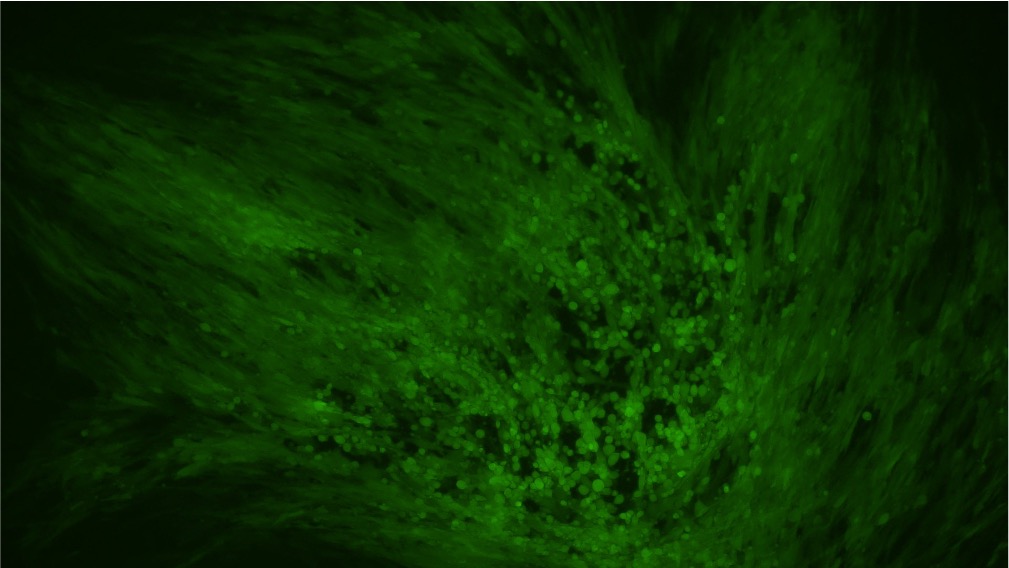
Figure 3. BHK21 cells were transduced with VACV produced from our BACYAC vector carrying an EGFP reporter at an MOI of 0.5. Images were taken at 0 hours and 72 hours post-transduction. Magnification: 100x. Left: bright field. Right: EGFP.
Vaccinia background
VACV is a double-stranded linear DNA virus belonging to the poxvirus family. Its genome length is about 190 kb and it can incorporate large transgenes (25-30 kb). Replication and transcription of the virus occurs completely in the cytoplasm of host cells, and the virus genome encodes all proteins needed for DNA replication and RNA transcription. Therefore, there is minimal dependence on the host cell machinery for the virus’s life cycle.
At the early stage of the virus life cycle, many immunomodulatory proteins are produced, helping the virus to escape from the host innate antiviral defense. The mature VACV has two major forms: the intracellular mature virus (IMV), which is released upon cell lysis, and the extracellular enveloped virus (EEV), which is released from the cell via exocytosis. Both of these forms are able to infect host cells via fusion of the lipid membrane with the host cell membrane, bypassing the requirement of host cell surface receptor binding for infection.
Major applications of VACV vectors
Recombinant VACV vectors are used for a variety of gene therapy applications. They are a promising system due to many advantages including the capacity for a favorable safety profile and the ability to harbor large foreign genes, to infect a broad spectrum of host cells, and to enhance a host immune response while preventing immune targeting of the virus itself.
Within the Vaccinia family, various strains have been developed with different levels of infectiousness and capacity for replication, influencing their use in clinical applications. Two very common strains are the WR strain and the MVA strain. While MVA is more often used in clinical settings due to its attenuation, WR has a higher rate of replication and can modulate the host immune system to a greater degree. Both strains are currently being used widely in both research and clinical studies. Below are some of the major research areas where VACV has been used:
Cancer treatment
In addition to a direct ability to lyse cancer cells, VACV can also modulate the tumor microenvironment by recruiting immune cell to secrete cytokines to elicit an indirect anti-tumor immune response. In order to increase safety and efficacy while maintaining the inherent benefits of the virus, specific genetic modifications are made. For example, Pexa-Vec, an oncolytic virus, is attenuated through deletion of the TK gene and also expresses a GM-CSF transgene to increase the host immune response. This treatment has been investigated in clinical trials for a variety of solid tumors. Several other oncolytic VACV clinical trials are underway, highlighting the rapid growth of VACV as a system for oncolytic therapies.
Vaccine construction
VACV is a promising platform for delivery of foreign antigens. Their large capacity for packaging DNA and wide usage in smallpox vaccination have established them as an ideal candidate for the construction of vaccines against a wide variety of pathogens, including HIV and rabies.
Advantages of using VACV for gene therapy
Broad tropism: VACV does not have a specific surface receptor for cell entry, allowing it to infect a wide range of cells.
Minimal risk of genomic integration: VACV has a short life cycle of 8 hours. The viral replication takes place entirely in the cytoplasm, eliminating the risk of genome integration.
High efficacy in tumor cells: VACV can be designed to selectively infect cancer cells and replicate with high efficiency, leading to tumor cell lysis.
Independent viral replication: The virus depends very little on the host machinery for mRNA transcription, making it less influenced by biological changes of the host cell.
Host immune evasion: VACV is able to evade the host immune system through secretion of Vaccinia complement control protein, which binds to and inactivates host white blood cells, and the utilization of the extracellular enveloped form of the virus, which is encapsulated in a host-derived envelope. Therefore, VACV can be delivered remotely to target distant tissues, including tumors, through a variety of introduction methods.
Large cargo capacity: VACV has a large genome with the ability to house exogenous genes up to 30 kb.
High viral titer and infectiousness: VACV vectors can be packaged into high titer virus, and VACV is highly infectious, requiring a low MOI for efficient transduction.
How to Order
Resources
FAQ
VACV offers several distinct advantages: large carrying capacity, the ability to target a variety of dividing and non-dividing cells, the ability to evade the host immune system, and viral replication occurring solely in the cytoplasm, minimizing the risk of genomic integration and decreasing reliance of host machinery. Further detailed comparisons with other virus types are laid out below.
| Lentivirus | MMLV | Adenovirus | AAV | VACV | |
|---|---|---|---|---|---|
| Tropism | Broad | Broad | Ineffective for some cells | Depending on viral serotype | Broad |
| Carrying capacity | 6.4 kb | 5.5 kb | 7.5 kb | 4.2 kb | 25-30 kb |
| Can infect non-dividing cells? | Yes | No | Yes | Yes | Yes |
| Stable integration or transient | Stable integration | Stable integration | Transient, episomal | Transient, episomal | Transient, episomal |
| Maximum titer | High | Moderate | Very High | High | High |
| Promoter customization | Yes | No | Yes | Yes | No |
| Primary use | Cell culture and in vivo | Cell culture and in vivo | In vivo | In vivo | Cell culture and in vivo |
| Immune response in vivo | Low | Low | High | Very low | High |
VACV vectors are not able to directly produce live virus but can be transfected into packaging cells that are infected with wild-type pox virus for live viral production. VectorBuilder has optimized this packaging process for high titers produced safely and efficiently.
The VACV requires Biosafety Level 2 facilities.




