Adeno-Associated Virus (AAV) Packaging
Recombinant adeno-associated virus (AAV) is a versatile and popular viral vector used for in vitro and in vivo gene delivery. AAVs have emerged as one of the most effective vehicles for gene therapy due to their ability to transduce a wide variety of mammalian cell types and their non-pathogenicity and low immunogenicity in human.
VectorBuilder offers superior quality AAV packaging services to support your AAV-based gene therapy experiments. We have developed a series of proprietary technologies and reagents that have greatly improved recombinant AAV production protocols in terms of titer, purity, potency and consistency, especially for the AAV vector systems used in our vector cloning services. As a result, we have a growing base of highly satisfied customers who come back to us time after time for their AAV vector cloning and AAV packaging needs. In addition to research-grade AAV, we also offer GMP-grade AAV manufacturing for clinical applications.
Types of AAV offered
- Single-stranded AAV (ssAAV) and self-complementary AAV (scAAV)
- Various serotypes: 1, 2, 3, 4, 5, 6, 6.2, 7, 8, 9, rh10, PHP.eB, PHP.S, AAV2-retro, AAV2-QuadYF, AAV2.7m8, etc.
- AAV empty capsids or virus-like particles (VLPs)
Service Details
Research grade AAV packaging
Our research grade AAV packaging services meet the vast majority of AAV-based gene delivery needs in basic research. Both triple transfection and baculovirus-based packaging methods can be selected based on your needs.
Triple Transfection-Based Approach
Baculovirus-Based Approach
Price and turnaround Price Match
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Pilot | Non-purified, suitable for most cell culture experiments | >1012 GC/ml | >2x1011 GC/ml | 250 ul (10x25 ul) | $449 |
6-12 days
|
| Medium | 1ml (10x100 ul) | $649 | ||||
| Large | >5x1012 GC/ml | >2x1012 GC/ml | 1ml (10x100 ul) | $1,099 | ||
| Ultra-purified pilot | Cell culture & in vivo | >2x1013 GC/ml | >1013 GC/ml | 100 ul (4x25 ul) | $1,399 |
7-14 days
|
| Ultra-purified medium | 500 ul (10x50 ul) | $1,999 | ||||
| Ultra-purified large | 1 ml (10x100 ul) | $3,099 | ||||
| Ultra-purified large 5 | 5 ml (10×500 ul) | From $9,899 | 14-21 days | |||
| Ultra-purified large 10 | 10 ml (10×1 ml) | From $15,899 | 21-28 days | |||
| Other scales | Please inquire | |||||
Note:
1. GC = Genome copies.
2. AAV serotypes 3 and 4 tend to have lower yields than other serotypes. As such, we can only guarantee 50% of the minimum titers specified in the table for these serotypes.
3. For ultra-purified scales, viral particles are purified by cesium chloride (CsCl) density gradient.
Deliverables
| For non-purified scales | For ultra-purified scales |
|---|---|
| Your custom AAV | Your custom AAV |
|
Free: non-purified control virus
|
Add-on purchase (optional): ultra-purified control virus
|
Control virus
The control AAV is designed to match the biological application of the custom virus and to be used for testing AAV transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control AAV (AAV overexpressing EGFP), and if the custom virus expresses an shRNA against a gene, then the control virus provided will express a scramble shRNA. Detailed information on the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| ssAAV gene expression system | pAAV[Exp]-CAG>EGFP:WPRE | VB010000-9287ffw |
| scAAV gene expression system | pscAAV[Exp]-CMV>EGFP | VB010000-9304aud |
| ssAAV U6-based shRNA knockdown system | pAAV[shRNA]-CAG>EGFP-U6>Scramble_shRNA | VB010000-9489hhg |
| scAAV U6-based shRNA knockdown system | pscAAV[shRNA]-EGFP-U6>Scramble_shRNA | VB010000-9343nhh |
| ssAAV miR30-based shRNA knockdown system | pAAV[miR30]-CAG>EGFP:Scramble_miR30-shRNA:WPRE | VB010000-9494mnd |
Research plus grade AAV packaging NEW
Our research plus AAV packaging services are suitable for applications that are sensitive to impurities (e.g. host cell protein, endotoxin, etc.), or applications with special requirements on purification method, titer, or formulation. They are also optimal choices for your preclinical animal experiments.
Price and turnaround Price Match
| Scale | Application | Total Yield (GC) | Price (USD) | Turnaround |
|---|---|---|---|---|
| Research-plus 1 | Various in vitro & in vivo experiments | 1x1013 | From $4,699 | 10-20 days |
| Research-plus 5 | 5x1013 | From $14,899 | 21-28 days | |
| Research-plus 10 | 1x1014 | From $23,899 | ||
| Other scales | Please inquire | |||
Note:
1. GC = Genome copies.
2. The above listed price and turnaround are based on purification by CsCl density gradient centrifugation. When other purification methods (e.g. iodixanol density gradient, affinity chromatography, ion-exchange chromatography, etc.) are required, please send us a design request to get the quote.
Price and turnaround Price Match
| Scale | Application | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|
| Ultra-purified pilot | Cell culture & in vivo | >5x1013 GC/ml | 1 ml (10x100 ul) | $5,599 | 35-49 days |
| Ultra-purified medium | 5 ml (25x200 ul) | $20,199 | 35-49 days | ||
| Ultra-purified large | 10 ml (50x200 ul) | $38,199 | 35-49 days |
Note:
1. GC = Genome copies.
2. Baculovirus-based approach is available for the following serotypes: 1, 2, 5, 6, 8, 9.
3. For ultra-purified scales, viral particles are purified by cesium chloride (CsCl) density gradient.
Deliverables
| Your custom AAV |
|
Add-on purchase (optional): ultra-purified control virus
|
Control virus
The control AAV is designed to match the biological application of the custom virus and to be used for testing AAV transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control AAV (AAV overexpressing EGFP), Detailed information on the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| Chimeric baculovirus-AAV gene expression system | pBV-ITR-CMV>EGFP-ITR | VB010000-9306ded |
Shipping and storage
Our non-purified AAV is stored in a Tris-based buffer and our ultra-purified AAV is stored in a PBS-based buffer. All our AAVs are shipped on dry ice. Upon receiving, it should be stored at -80°C for long term (stable for at least 1 year), or -20°C for short term, e.g. 2-3 weeks. Thawed AAV virus can be stored at 4 °C for 1-2 weeks. Although AAV is more stable than many other virus (e.g. lentivirus) and can be frozen and thawed several times with minimal loss of virus activity, it is best to avoid repeated freeze-thaw cycles in practice.
Technical Information
Comparison of different AAV grades
The table below is an overview comparison of different grades of AAV we offer for research use.
| Non-purified research grade | Ultra-purified research grade | Research plus grade | |
|---|---|---|---|
| Available purification method | - | CsCl density gradient | CsCl density gradient (default), iodixanol density gradient, affinity chromatography, ion-exchange chromatography |
| Titer | >1012 GC/ml | >1013 GC/ml | 1x1013 - 5x1013 GC/ml |
| Achievable purity (assessed by SDS-PAGE) | - | >80% | >90% |
| Achievable endotoxin level | <30 EU/ml | <10 EU/ml | <2 EU/ml |
| Typical full capsid ratio | - | >70% | >80% |
AAV production and quality control (QC)
Triple Transfection-Based Approach
Baculovirus-Based Approach
For our recombinant AAV manufacturing, the transfer plasmid carrying the gene of interest (GOI) is co-transfected with our proprietary Rep-cap plasmid and helper plasmid encoding adenovirus genes (E4, E2A and VA) that mediate AAV replication into HEK293T packaging cells. After a short incubation period, viral particles are harvested from cell lysate or supernatant depending on serotype and concentrated by PEG precipitation. For ultra-purified AAV (in vivo grade), viral particles are further purified and concentrated by cesium chloride (CsCl) gradient ultracentrifugation. We use a qPCR-based approach to measure AAV titer.
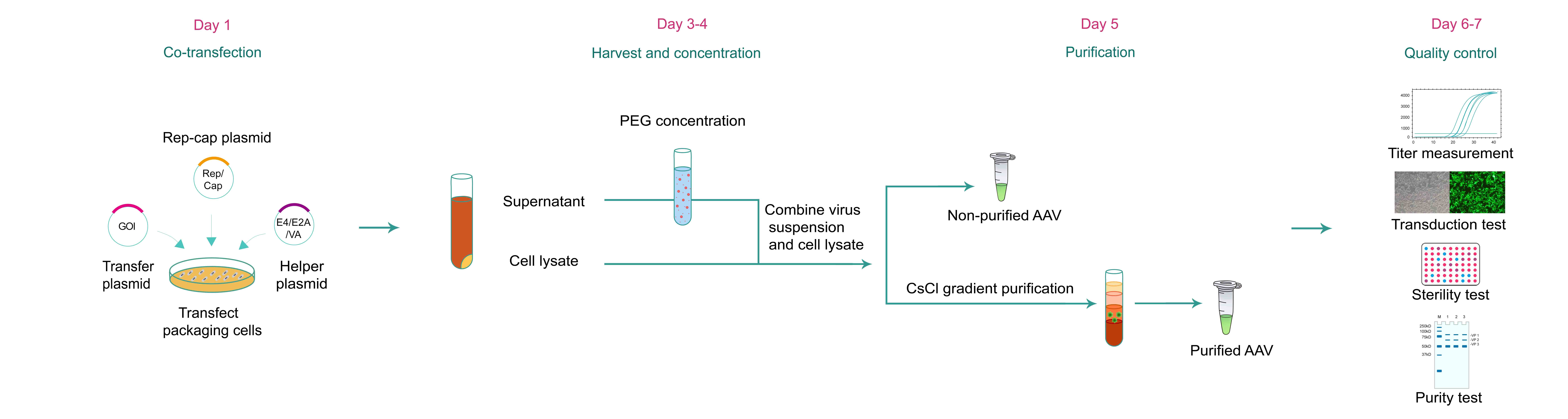
Figure 1. Typical workflow of triple transfection-based AAV packaging
For each AAV produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the transfer vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. Additionally, for ultra-purified AAV, we routinely sample virus quality by SDS-PAGE analysis and endotoxin assay. To include the endotoxin results in your COA, an extra cost is required. Additional QC services as below can be provided upon request.
| Additional QC service | Method |
|---|---|
| Endotoxin testing | LAL |
| Titer determination | ddPCR |
| TCID50 | |
| Replication-competent AAV testing | qPCR |
| Full/empty capsid analysis | TEM |
| CDMS | |
| SV-AUC |
Our baculovirus-based AAV packaging workflow consists of two main steps as shown in Figure 1 below. Step 1 involves the generation of two recombinant baculoviruses, specifically the first one expressing the gene of interest (GOI) flanked by the AAV inverted terminal repeats (ITRs) and a second helper baculovirus expressing the AAV rep and cap genes. In step 2 recombinant AAV particles are generated by co-infecting insect cells with the two recombinant baculoviruses produced in step 1.
For generating the two recombinant baculoviruses (one expressing the GOI flanked by AAV ITRs and the other expressing the AAV rep/cap genes), the expression cassette for each baculovirus is first cloned into a baculovirus transfer vector. The baculovirus transfer plasmid is then co-transformed with a helper plasmid expressing the Tn7 transposase into the bacterial host harboring the empty baculovirus shuttle vector (a.k.a. bacmid) to generate recombinant bacmid. The recombinant bacmid is then transfected into insect Sf9 cells. After a short incubation period, viral particles are harvested from medium and further concentrated by sucrose cushion centrifugation. We use a qPCR-based approach to measure baculovirus titer. Both recombinant baculoviruses are then co-infected into insect cells. After an incubation period of 72-96 hours, AAV particles are harvested from the cell lysate as well as the supernatant and concentrated by PEG precipitation. For ultra-purified AAV (in vivo grade), viral particles are further purified and concentrated by cesium chloride (CsCl) gradient ultracentrifugation. We use a qPCR-based approach to measure AAV titer.
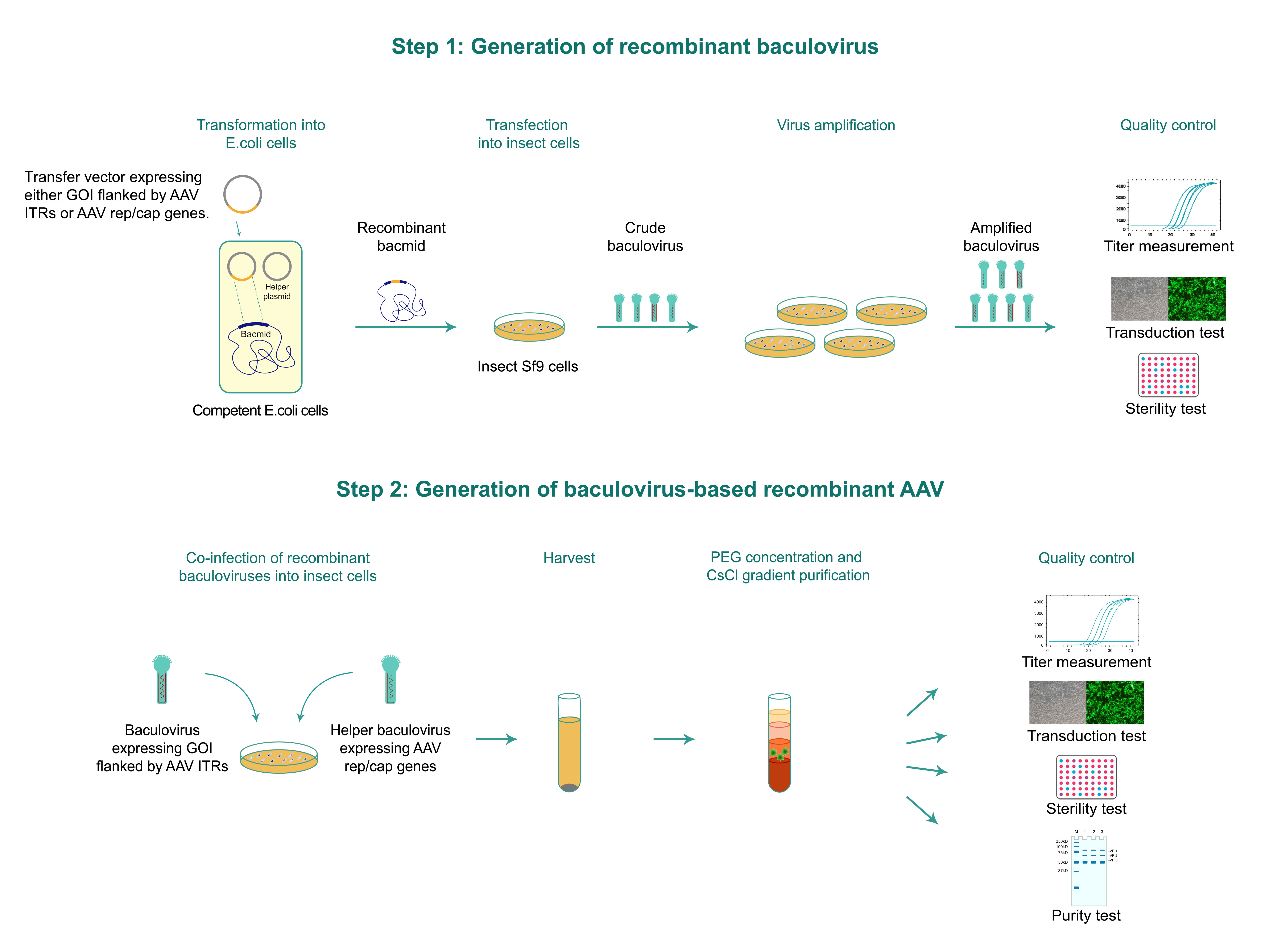
Figure 1. Typical workflow of baculovirus-based AAV packaging
For each AAV produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the transfer vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. Additionally, for ultra-purified AAV, we routinely sample virus quality by SDS-PAGE analysis and endotoxin assay. Extra cost is required for including endotoxin result in your COA report. Additional QC services as below can be provided upon request.
| Other QC services | Methods |
|---|---|
| Titer determination | ddPCR |
| TCID50 | |
| Replication-competent AAV testing | qPCR |
| Empty capsid analysis | TEM |
| CDMS | |
| SEC-AUC |
Recommended AAV serotypes
List by Serotype
List by Tissue Type
| Serotype | Tissue tropism |
|---|---|
| AAV1 | Smooth muscle, skeletal muscle, CNS, brain, lung, retina, inner ear, pancreas, heart, liver |
| AAV2 | Smooth muscle, CNS, brain, liver, pancreas, kidney, retina, inner ear, testes |
| AAV3 | Smooth muscle, liver, lung |
| AAV4 | CNS, retina, lung, kidney, heart |
| AAV5 | Smooth muscle, CNS, brain, lung, retina, heart |
| AAV6 | Smooth muscle, heart, lung, pancreas, adipose, liver |
| AAV6.2 | Lung, liver, inner ear |
| AAV7 | Smooth muscle, retina, CNS, brain, liver |
| AAV8 | Smooth muscle, CNS, brain, retina, inner ear, liver, pancreas, heart, kidney, adipose |
| AAV9 | Smooth muscle, skeletal muscle, lung, liver, heart, pancreas, CNS, brain, retina, inner ear, testes, kidney, adipose |
| AAV-rh10 | Smooth muscle, lung, liver, heart, pancreas, CNS, retina, kidney |
| AAV-DJ | Liver, heart, kidney, spleen |
| AAV-DJ/8 | Liver, brain, spleen, kidney |
| AAV-PHP.eB | CNS |
| AAV-PHP.S | PNS |
| AAV2-retro | Spinal nerves |
| AAV2-QuadYF | Endothelial cell, retina |
| AAV2.7m8 | Retina, inner ear |
| Tissue type | Recommended AAV serotypes |
|---|---|
| Smooth muscle | AAV1, AAV2, AAV3, AAV5, AAV6, AAV7, AAV8, AAV9, AAV-rh10 |
| Skeletal muscle | AAV1, AAV9 |
| CNS | AAV1, AAV2, AAV4, AAV5, AAV7, AAV8, AAV9, AAV-rh10, AAV-PHP.eB |
| PNS | AAV-PHP.S |
| Brain | AAV1, AAV2, AAV5, AAV7, AAV8, AAV9, AAV-DJ/8 |
| Retina | AAV1, AAV2, AAV4, AAV5, AAV7, AAV8, AAV9, AAV-rh10, AAV2-QuadYF, AAV2.7m8 |
| Inner ear | AAV1, AAV2, AAV6.2, AAV8, AAV9, AAV2.7m8 |
| Lung | AAV1, AAV3, AAV4, AAV5, AAV6, AAV6.2, AAV9, AAV-rh10 |
| Liver | AAV1, AAV2, AAV3, AAV6, AAV6.2, AAV7, AAV8, AAV9, AAV-rh10, AAV-DJ, AAV-DJ/8 |
| Pancreas | AAV1, AAV2, AAV6, AAV8, AAV9, AAV-rh10 |
| Heart | AAV1, AAV4, AAV5, AAV6, AAV8, AAV9, AAV-rh10, AAV-DJ |
| Kidney | AAV2, AAV4, AAV8, AAV9, AAV-rh10, AAV-DJ, AAV-DJ/8 |
| Adipose | AAV6, AAV8, AAV9 |
| Testes | AAV2, AAV9 |
| Spleen | AAV-DJ, AAV-DJ/8 |
| Spinal nerves | AAV2-retro |
| Endothelial cells | AAV2-QuadYF |
* Please note that the ITRs carrying your gene of interest (GOI) is from the AAV2 genome. Different serotypes are distinguished by the capsid protein serotype.
Click here to view the amino acid sequence of the major viral capsid protein 1 (VP1) for different AAV serotypes >>Experimental validation
We have developed a number of proprietary techniques to optimize our baculovirus-based AAV packaging protocol and our virus has been validated to exhibit high transduction efficiency in mammalian cells.
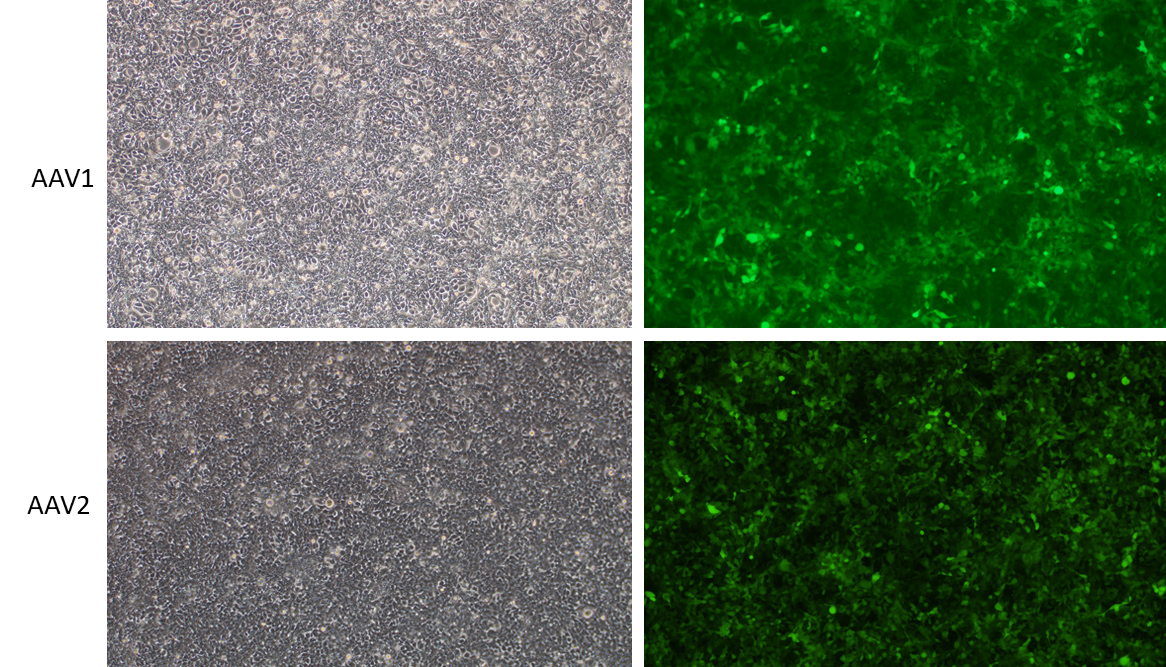
Figure 2. HEK293T cells were transduced with EGFP expressing, baculovirus-based AAV1 and AAV2 at MOI 10000. Magnification: 100x. Left: bright field. Right: EGFP.
Experimental validation
We have developed a number of proprietary techniques to optimize our triple transfection-based AAV packaging protocol and our virus has been validated to exhibit high transduction efficiency in mammalian cells.
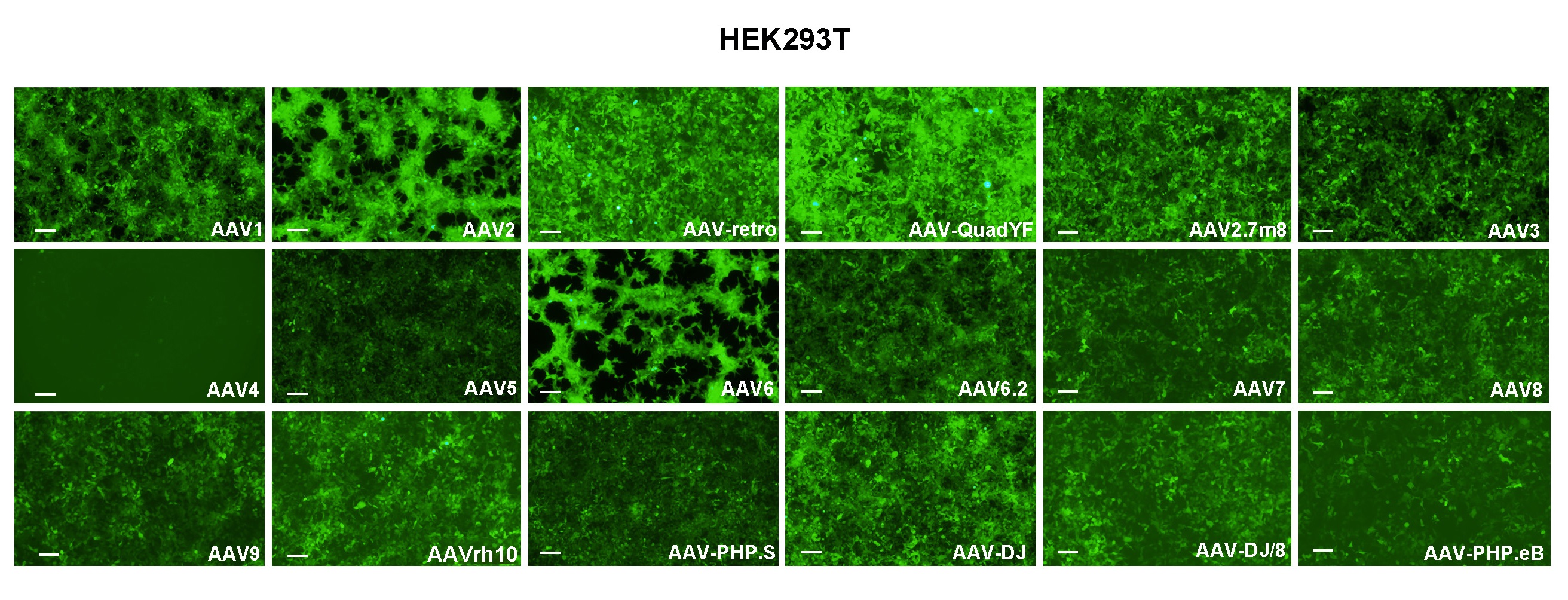
Figure 2. HEK293T cells were transduced with 18 serotypes of recombinant AAV packaged from the same CMV>EGFP vector (VB010000-9394npt). Representative EGFP expression at 48 h post transduction in different serotypes is shown as indicated. Scale bars: 100 μm.
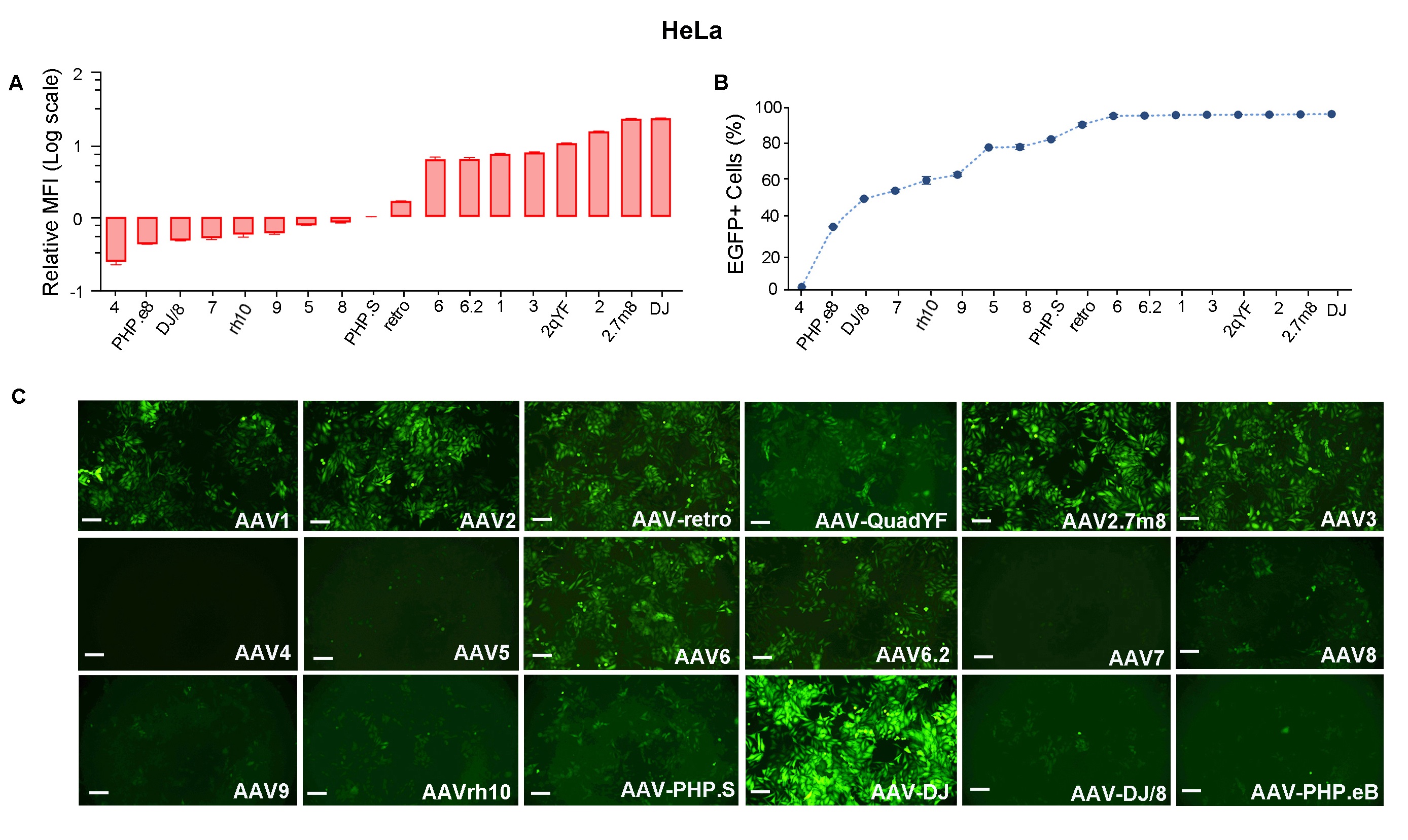
Figure 3. HeLa cells were transduced with 18 serotypes of recombinant AAV packaged from the same CMV>EGFP vector (VB010000-9394npt). EGFP (A) Mean Fluorescence Intensity (MFI) and (B) positivity were quantified using flow cytometry 48 h post transduction. (C) Representative EGFP expression in different serotypes is shown as indicated. Scale bars: 100 μm.
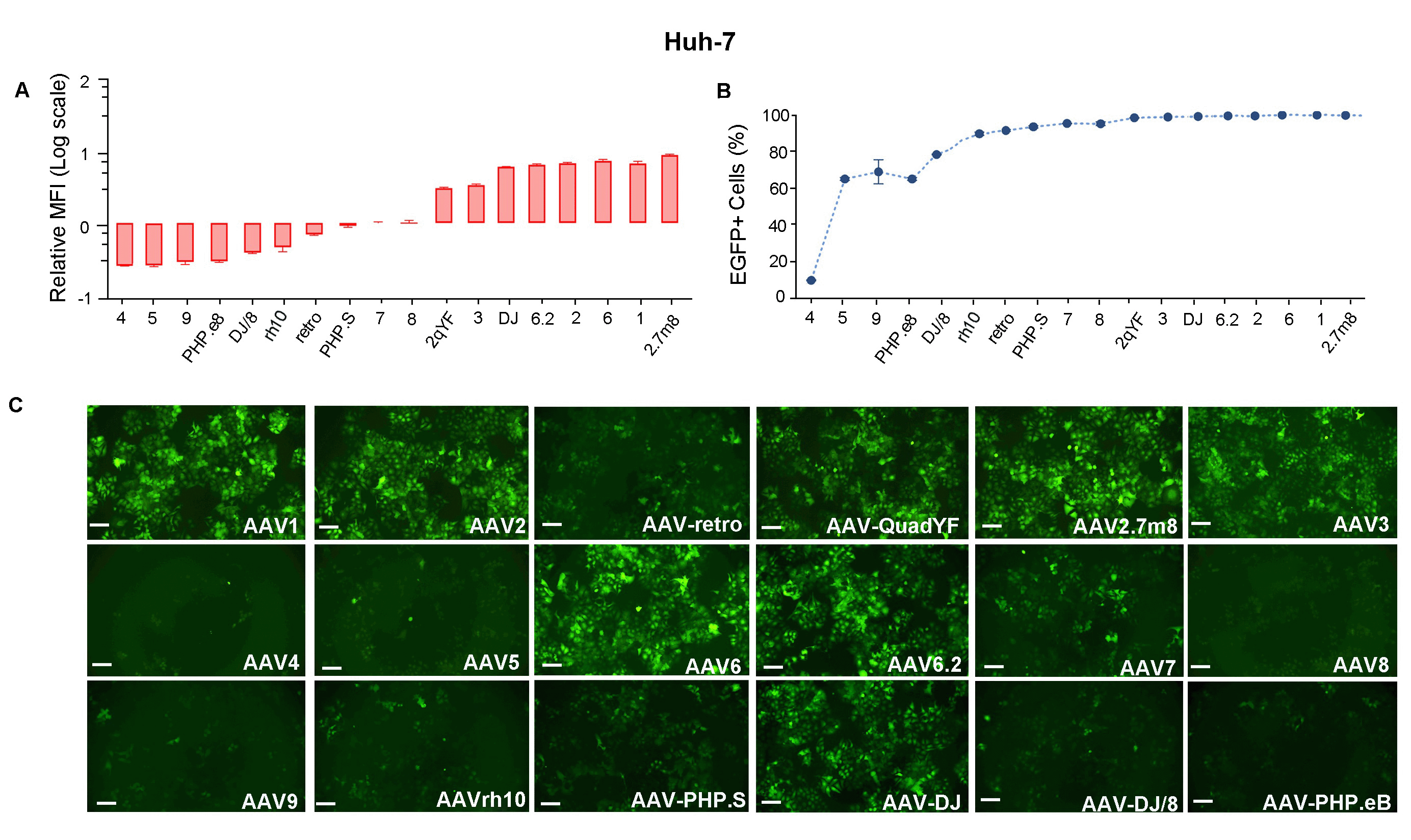
Figure 4. Huh-7 cells were transduced with 18 serotypes of recombinant AAV packaged from the same CMV>EGFP vector (VB010000-9394npt). EGFP (A) Mean Fluorescence Intensity (MFI) and (B) positivity were quantified using flow cytometry 48 h post transduction. (C) Representative EGFP expression in different serotypes is shown as indicated. Scale bars: 100 μm.
How to Order
Customer-supplied vectors
If customer-supplied AAV plasmids are used in packaging, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
Resources
Documents
User Instructions Material Safety Data Sheet (MSDS)- Non-purified recombinant adeno-associated virus particles
- Ultra-purified recombinant adeno-associated virus particles
FAQ
| Lentivirus | MMLV | Adenovirus | AAV | |
|---|---|---|---|---|
| Tropism | Broad | Broad | Ineffective for some cells | Depending on viral serotype |
| Can infect non-dividing cells? | Yes | No | Yes | Yes |
| Stable integration or transient | Stable integration | Stable integration | Transient, episomal | Transient, episomal |
| Maximum titer | High | Moderate | High | Very high |
| Promoter customization | Yes | No | Yes | Yes |
| Primary use | Cell culture and in vivo | Cell culture and in vivo | In vivo | In vivo |
| Immune response in vivo | Low | Low | High | Very low |
We measure the physical titer of AAV by directly extracting viral genome from lysed viral particles, and then using qPCR to accurately quantify the copy number of viral genome (using the copy number of ITR region as a proxy) in the stock. Titration services using other methods (e.g. ddPCR, TCID50) can be purchased separately.
Our titer guarantee applies to vectors for which the region being packaged into virus (from 5’ ITR to 3’ ITR) is below the AAV cargo limit (4.7 kb). For sizes above this limit, the region packaged into the virus may be truncated and result in loss-of-function. Additionally, we may not be able to guarantee titer in the following situations:
- AAV serotypes 3 and 4 tend to have lower yields than other serotypes. As such, we can only guarantee 50% of the minimum titers of our standard AAV packaging scales.
- Vectors containing sequences that could adversely affect the packaging process such as toxic genes (e.g. proapoptotic genes), genes that compromise the integrity of packaging cells or virus (e.g. membrane proteins that cause cell aggregation), and sequences prone to rearrangements or secondary structures (e.g. repetitive or highly GC-rich sequences).
- Packaging of customer-supplied vector because we have no control over the quality of the vector and the compatibility of the vector with our packaging system.
Our estimated turnaround is the time from production initiation to completion. It does not include waiting time for any customer-supplied materials (e.g. template DNA or viral vectors), QC of such materials, and transit time for shipping final deliverables to the customer.