Inducible Gene Expression Solutions
VectorBuilder offers a comprehensive collection of Tet inducible gene expression system reagents to help you achieve tetracycline-regulated expression of your target genes. We provide a wide range of pre-made and custom-made vectors with non-viral, viral and transposon backbones, tailoring to your experimental goals. We can package all major virus types (e.g. lentivirus, AAV, adenovirus) at various titer scales for delivering Tet system components into difficult-to-transfect cells. We can also generate Tet inducible gene expression stable cells lines for efficient and reliable induction of your genes of interest (GOIs) by tetracycline or its analogs (e.g. doxycycline).
Highlights
- Available in all-in-one and dual vector formats
- Utilizes the tTS/rtTA fusion cassette as the true on-and-off switch to achieve robust induction with minimal leaky expression
- Low-leak tissue-specific vectors available with minimal leaky expression in non-target tissues
- 100% sequence validated, fast turnaround and competitive pricing
- Powerful technical support for experimental design, data analysis and troubleshooting
Service Details Price Match
Custom Tet vectors
VectorBuilder offers a variety of custom vectors for efficient delivery of tetracycline inducible gene expression cassettes into target cells. Using our highly intuitive online vector design platform, you can choose from a wide collection of vector backbones (non-viral, viral or transposon) and unlimited combinations of vector components (promoters, ORFs, fluorescent and drug-selection markers) for designing custom vectors to achieve Tet regulated expression of your GOI(s).
Plasmid DNA preparation and virus packaging can be purchased as downstream services when adding custom Tet vectors into shopping cart.
Design tips
- TRE driven GOI expression vector and Tet regulatory protein expression vector are NOT all-in-one Tet vectors. To achieve Tet regulated gene expression, you need the expression of both your GOI driven by TRE promoter and Tet regulatory protein in your cells of interest.
- In the 2nd generation Tet system, TRE promoter is used and it can interact with Tet regulatory proteins such as rtTA, tTS and tTA. In the 3rd generation Tet system, TRE3G promoter is used and the corresponding Tet regulatory protein is Tet3G.
- When designing a dual vector system, select two different markers for TRE driven GOI expression vector and Tet regulatory protein expression vector. If your experimental system already contains a marker (e.g. cells of interest expressing an antibiotic resistant gene), check for its compatibility with the markers on your Tet vectors.
Choose your custom Tet vectors View more
Popular Tet vectors
VectorBuilder offers a panel of popular Tet vectors in both all-in-one and dual vector formats. Plasmid DNA preparation and virus packaging can be purchased as downstream services when adding popular Tet vectors into shopping cart.
You can use the vector pickers below for ordering popular Tet vectors.
Selection tips
- TRE driven GOI expression vector and Tet regulatory protein expression vector are NOT all-in-one Tet vectors. To achieve Tet regulated gene expression, you need the expression of both your GOI driven by TRE promoter and Tet regulatory protein in your cells of interest.
- Select a Tet regulatory protein expression vector for generating stable cell lines expressing tTA, tTS and/or rtTA.
All-in-one (Tet-on)
Note: If you need lentiviral all-in-one (Tet-on) vectors, please send us a design request.
TRE driven GOI expression
Tet regulatory protein expression
If you unable to find your desired Tet vectors using the vector pickers above, simply send us a design request describing your needs and our scientists will assist you from there.
Virus packaging for Tet vectors
VectorBuilder offers premium quality virus packaging services for lentivirus, AAV and adenovirus in a variety of scales for delivering Tet-inducible gene expression system into difficult-to-transfect cells. Our proprietary technologies and reagents have greatly improved virus packaging protocols in terms of titer, purity, viability and consistency. Our packaging protocols are also optimized for the viral vector systems used in our vector construction services. As a result, we have a growing base of highly satisfied customers who come back to us again and again for their cloning and virus packaging needs.
Price, turnaround and scales of lentivirus packaging services View more
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Mini | Cell culture | >2x108 TU/ml | >108 TU/ml | 100 ul (4x25 ul) | $199 | 6-12 days |
| Pilot | >4x108 TU/ml | 250 ul (10x25 ul) | $449 | |||
| Medium | >3x108 TU/ml | 1 ml (10x100 ul) | $649 | |||
| Large | >2x109 TU/ml | >109 TU/ml | 1 ml (10x100 ul) | $1,099 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x109 TU/ml | >109 TU/ml | 500 ul (10x50 ul) | $1,399 | |
| Ultra-purified large | 1 ml (10x 100 ul) | $1,699 |
TU = Transduction units (also known as infectious units)
Price, turnaround and scales of AAV packaging servicesView more
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Pilot | Cell culture | >1012 GC/ml | >2x1011 GC/ml | 250 ul (10x25 ul) | $449 | 6-12 days |
| Medium | 1 ml (10x100 ul) | $649 | ||||
| Large | >5x1012 GC/ml | >2x1012 GC/ml | 1 ml (10x100 ul) | $1,099 | ||
| Ultra-purified pilot | Cell culture & in vivo | >2x1013 GC/ml | >1013 GC/ml | 100 ul (4x25 ul) | $1,399 | 7-14 days |
| Ultra-purified medium | 500 ul (10x50 ul) | $1,999 | ||||
| Ultra-purified large | 1 ml (10x100 ul) | $3,099 |
GC = Genome copies
Price, turnaround and scales of adenovirus packaging services View more
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Pilot | Cell culture | >2x1010 IFU/ml | >1010 IFU/ml | 250 ul (10x25 ul) | $649 | 28-35 days |
| Medium | 1 ml (10x100 ul) | $1,099 | ||||
| Large | >2x1011 IFU/ml | >1011 IFU/ml | 1 ml (10x100 ul) | $1,699 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x1012 VP/ml | >1012 VP/ml | 500 ul (10x50 ul) | $2,099 | |
| Ultra-purified large | 1 ml (10x100 ul) | $2,499 |
IFU = Infectious units; VP = Virus particles
Click to view detailed information on our virus packaging services
Tet inducible gene expression stable cell lines
VectorBuilder can custom build Tet inducible gene expression stable cell lines with minimal background expression and high induction of your GOI. Additionally, we can generate tTS/rtTA expressing cell lines which can then be transfected/transduced with plasmids/viruses carrying TRE driven GOI(s) for flexible experimental design and reliable gene induction. For stable Tet-On cell lines, induction of the GOI is validated by RT-qPCR. Additionally, a series of standard QC assays such as sterility tests and mycoplasma detection are performed for releasing the final cell line products.
Price and turnaround of our Tet inducible gene expression stable cell line services View more
| Stable Cell Line Model | Strategy | Deliverable | Price (USD) | Turnaround |
|---|---|---|---|---|
| tTS/rtTA Expressing Stable Cell Line | Lentivirus-based | Pooled Cells | From $2,199 | 7-9 weeks |
| 3 Single Clones | From $4,199 | 9-15 weeks | ||
| Tet-On Gene Expression Stable Cell Line | Lentivirus-based | Pooled Cells | From $3,599 | 10-16 weeks |
| 3 Single Clones | From $5,899 | 13-21 weeks |
Click here for detailed information on our stable cell line generation services
Technical Information
Tetracycline inducible gene expression
The tetracycline inducible gene expression system is a powerful tool to control the timing of gene expression in mammalian cells (Figure 1). We offer both Tet-On and Tet-Off systems to help you achieve highly specific, tetracycline dose-dependent regulation of your GOI expression.
Our Tet-On vector systems are designed to achieve nearly complete silencing of a GOI in the absence of tetracycline, and strong, rapid expression in response to the addition of tetracycline. This is achieved through a multicomponent system which incorporates active silencing by the tTS protein in the absence of tetracycline and strong activation by the rtTA protein in the presence of tetracycline. In the absence of tetracycline, the tTS protein derived from the fusion of TetR (Tet repressor protein) and KRAB-AB (the transcriptional repressor domain of Kid-1 protein) binds to the TRE promoter, leading to the active suppression of gene transcription. The rtTA protein, on the other hand, derived from the fusion of a rTetR (a mutant Tet repressor) and VP16 (the transcription activator domain of virion protein 16 of herpes simplex virus), binds to the TRE promoter to activate gene transcription only in the presence of tetracycline. Our Tet-Off vector system can be used for inhibiting target gene expression in the presence of tetracycline regulated by the binding of the tTA protein to the TRE promoter.
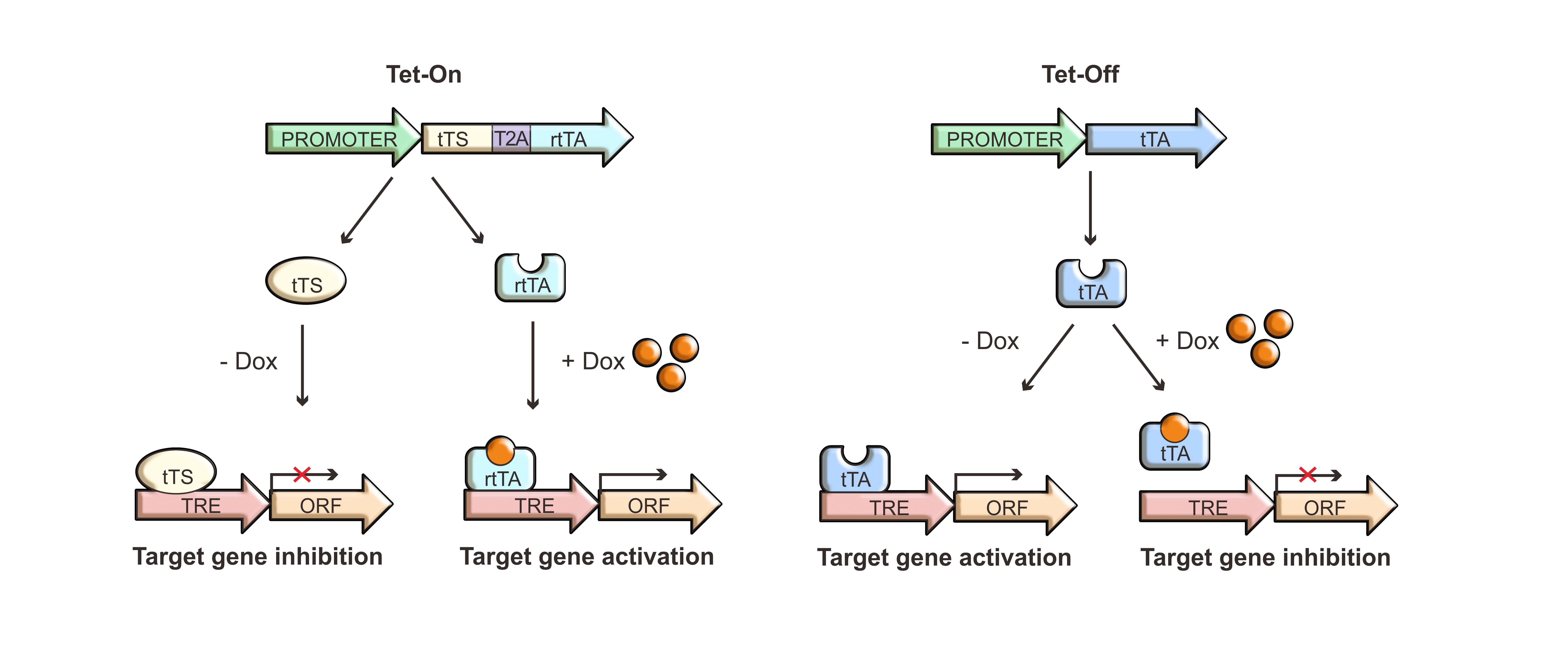
Figure 1. Mechanisms of Tet regulated gene expression using Tet-On and Tet-Off systems. Dox: doxycycline (a tetracycline analog)
The Tet inducible gene expression system has gained popularity over other available inducible gene expression systems since it offers several advantages over other systems which are summarized in the table below:
| Tet Inducible Gene Expression System | Other Inducible Gene Expression Systems | |
|---|---|---|
| Regulation | Minimized leaky expression in the absence of tetracycline. | High levels of leaky expression in the absence of inducer due to basal transgene expression. E.g. Tamoxifen-based system, riboswitch-based system. |
| Gene induction level | Achieves rapid and high levels (1000-fold) of target gene induction in the presence of tetracycline. | Achieves much lower levels (100-fold) of target gene induction. E.g. Tamoxifen-based system, riboswitch-based system. |
| Reversibility | Can achieve reversible gene expression by the addition and removal of tetracycline. | Results in irreversible activation of gene expression. E.g. Tamoxifen-based system. |
| Toxicity | High level induction can be achieved with non-toxic levels of doxycycline. | Exhibits increased cellular toxicity. E.g. Tamoxifen-based system. |
| Cost-effective | Requires tetracycline or one of its derivatives such as doxycycline which are cheap and easily available. | Requires inducers which are more expensive. E.g. hormone-based systems. |
| Pleiotropic effects | Minimized pleiotropic effects due to the absence of prokaryotic regulatory sequences in mammalian cells. | Increased pleiotropic effects due to activation of non-target endogenous genes. E.g. Ecdysone-based system, CID-based system. |
All-in-one vs. dual vector design
To achieve tetracycline regulated induction or inhibition of your GOI, it is essential for the target cells to co-express the GOI driven by the TRE promoter and the Tet regulatory proteins (tTA, tTS and/or rtTA) at the same time. This can be accomplished by either expressing both the GOI and the Tet regulatory proteins from the same vector (a.k.a. all-in-one vector) or by using separate vectors for driving GOI and Tet regulatory protein expression (dual vectors). The advantage of using an all-in-one vector is that it can deliver all the required components for Tet regulated gene expression to the cell using a single vector which is technically straight forward. Using separate vectors for co-expressing the GOI and the Tet regulatory proteins requires co-transfection/co-transduction of the target cells with two separate plasmids/viruses which can be technically challenging since not all cells will be transfected/transduced with both plasmids/viruses simultaneously. An alternative approach for using dual vectors is to transfect/transduce cells or organisms stably expressing the Tet regulatory proteins with the plasmid/virus carrying the desired GOI. However, this method can be considerably time-consuming and labor intensive. Despite its challenges, the dual vector system is sometimes preferred since it offers the flexibility of using the same Tet regulatory protein expressing plasmid, virus or cell line with multiple different GOI expressing plasmids/viruses.
Tissue-specific Tet inducible gene expression
VectorBuilder offers all-in-one (Tet-On) vectors in two formats, standard and low-leak to help you achieve tissue-specific induction of your GOI(s). While the standard Tet-On vector system expresses tTS and rtTA as a fusion protein driven by the same user-selected tissue-specific promoter, the low leak Tet-On vector system utilizes a ubiquitous promoter for driving tTS expression and a user-selected tissue-specific promoter for driving rtTA expression. The low leak design offers the advantage of minimizing leaky expression in non-target tissues in the absence of tetracycline while allowing tissue-specific induction of target transgenes in the presence of tetracycline.
VectorBuilder’s online “Resources” > “Learning Center” contains rich educational materials to facilitate you to successfully plan, execute and troubleshoot your Tet inducible gene expression experiments.
Click to read guides on Tet inducible vector systemsClick to read guides on Tet vector components
Resources
Documents
User InstructionsFAQ
Unlike Tet-On systems relying only on rtTA that usually have significant leaky expression without induction, our Tet-On vector system expressing tTS/rtTA acts as a true tetracycline-regulated on-and-off switch for controlling gene expression, which can minimize background expression without induction and result in high sensitivity and high dynamic range of tetracycline induction (Figure 2). In the absence of tetracycline, the tTS protein binds to the TRE promoter leading to active suppression of gene transcription and thereby, minimizing background expression. The rtTA protein, on the other hand, binds to the TRE promoter to activate gene transcription only in the presence of tetracycline.
- Dox+ Dox
rtTA
tTS/rtTA
tTS/rtTA
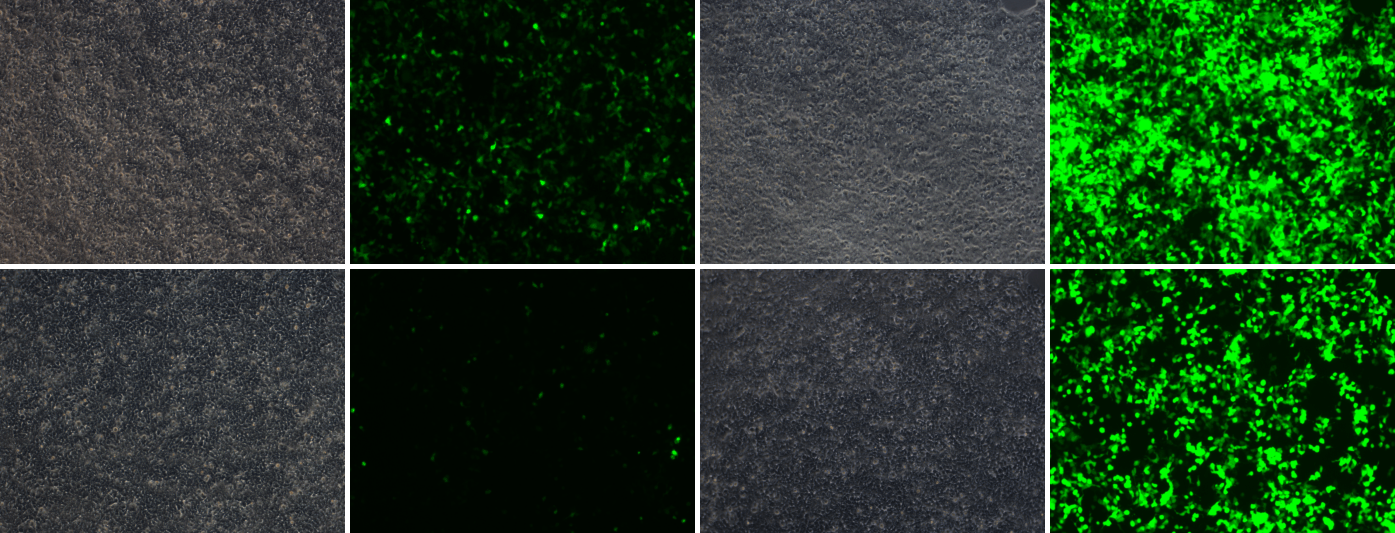
Figure 2. EGFP expression in 293T cells transfected with either an all-in-one (Tet-On) vector expressing EGFP and rtTA (Top) or an all-in-one (Tet-On) vector expressing EGFP and tTS/rtTA (Bottom). EGFP expression was checked both in the absence (-Dox) and presence (+Dox) of doxycycline 48 hours post-transfection.
For delivering Tet system components using lentivirus, we generally do not recommend all-in-one vector design. Based on our internal data and many of our customers’ feedback, the gene induction efficiency using all-in-one lentivirus is context-dependent and often not satisfying. An all-in-one vector usually contains two gene expression cassettes: GOI driven by TRE promoter, and Tet regulatory protein (e.g. tTA, tTS and/or rtTA) expression cassette. For lentiviral vector, carrying multiple gene expression cassettes are sometimes problematic due to the conflict between the requirement for proper virus packaging and the requirement for independent expression of different cassettes. Internal polyadenylation signal is not allowed to be placed between the two LTRs of lentiviral vector as this would interrupt virus packaging. As a result, transcription from the upstream promoter does not stop at the end of the upstream ORF, and continues to pass through the downstream promoter(s) and ORF(s), which often leads to partial inhibition of expression of the downstream ORF(s). In an all-in-one lentiviral vector, since Tet regulatory proteins are placed downstream of the GOI, their expression can be much reduced, leading to leaky expression and inefficient induction (Figure 3).
- Dox+ Dox
Dual
All-in-one
All-in-one
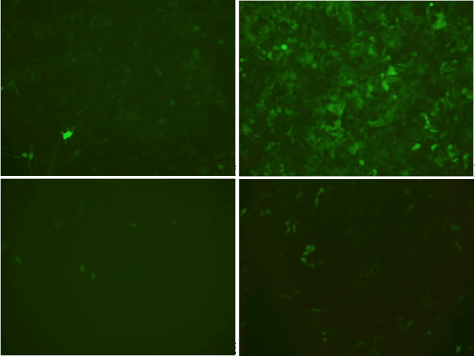
Figure 3. EGFP expression in 293T cells transduced with either dual (Tet-On) lentivirus (Top) or all-in-one (Tet-On) lentivirus (Bottom). For dual vector-based induction, 293T cells stably expressing tTS/rtTA were transduced with lentivirus expressing TRE driven EGFP. EGFP expression was checked both in the absence (-Dox) and presence (+Dox) of doxycycline 48 hours post-transduction.
One of the key factors underlying the design of any successful experiment is the choice of the vector system used for delivering the genes of interest into the target cells. Given that there are various viral and non-viral vector options available, several factors should be taken into consideration while selecting the ideal vector suitable for your experimental design. Some of the key considerations include: Are your target cells easy or difficult to transfect? Do you want transient expression or stable integration into the host genome? Do you need to use a customized promoter to drive your gene of interest? Will your vector be used in cell culture or in vivo? Do you need conditional or inducible gene expression? How big is your gene of interest?
The table below lists the commonly used vector systems and key considerations for selecting the right vector suitable for your experimental design.
| Regular Plasmid Vectors | Viral Vectors | Transposon-based Vectors | |
|---|---|---|---|
| Transfection-based | Yes | No | Yes |
| Transient expression or stable integration | Transient | Stable integration | Stable integration |
| Requires packaging | No | Yes | No |
| Cargo capacity | Large | Small to medium | Medium to large |
| Primary use | Cell culture | Cell culture & In vivo | Cell culture & in vivo |
| Promoter customization | Yes | Depending on viral vector type | Yes |



