Herpes Simplex Virus (HSV) Cloning and Packaging
Recombinant herpes simplex virus (HSV) has recently emerged as a promising viral vector in gene therapy and cancer therapy, with a variety of applications including gene delivery into the nervous system, as oncolytic agents, and for the development of vaccines against cancer, HSV and other infectious diseases. HSV vectors offer several advantages such as natural neurotropism, the ability to persist in a latent, episomal state throughout the lifetime of host cells, and their large cargo carrying capacity. These features make them the ideal viral vehicles for a range of in vitro and in vivo gene delivery needs.
VectorBuilder specializes in the design and construction of HSV vectors that can be used to reconstitute live viruses, including wildtype and attenuated viruses as well as replication-defective amplicons. We also provide packaging services for both research-grade and GMP-grade HSV.
Types of HSV services offered
VectorBuilder offers following services for HSV:
- HSV vector cloning in BAC or BACYAC backbone
- HSV-1 virus packaging
- HSV-1 amplicon vector cloning
Service Details
HSV BACYAC vector cloning
VectorBuilder has develop a proprietary “BACYAC” backbone for cloning HSV vectors. This backbone combines key elements from both the bacterial artificial chromosome (BAC) and the yeast artificial chromosome (YAC). The BAC elements on the backbone allow the vector to behave like a BAC and be propagated in E. coli, whereas the YAC elements allow the vector to behave like a YAC and be propagated in the yeast Saccharomyces cerevisiae. Our BACYAC backbone thus provides users with the flexibility to grow and modify the vector in either E. coli or yeast.
The BACYAC backbone is available with either LacZ or EGFP reporter that enables infected cells to be identified easily based on reporter expression. The backbone is flanked by a pair of loxP sites to facilitate removal of the backbone from the final viral genome by Cre-mediated recombination.
The basic design of our HSV BACYAC vectors is illustrated in Figure 1 below.
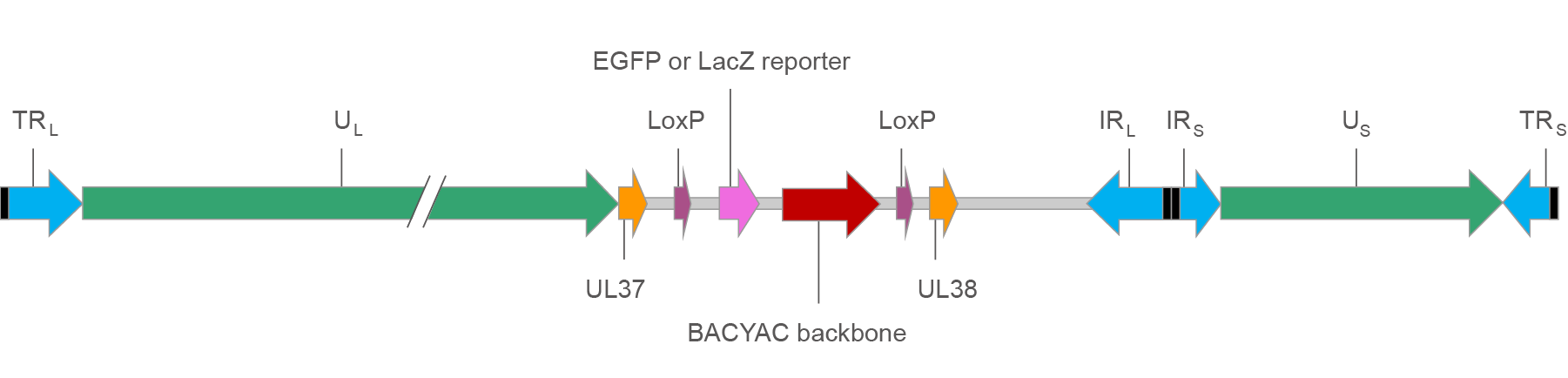
Figure 1. Map of HSV BACYAC with EGFP/LacZ reporter.
TRL/IRL: Terminal and inverted repeat regions flanking unique long segments. They are bounded by ‘a’ sequences (black boxes), at which recombination happens for 4 isomers.
TRS/IRS: Terminal and inverted repeat regions flanking unique short segments. They are bounded by ‘a’ sequences (black boxes), at which recombination happens for 4 isomers.
UL/US: Unique long and short segments encoding HSV genes.
UL37/UL38: HSV UL37 and UL38 genes. Insertion of foreign DNA into the intergenic region between UL37 and UL38 results in a small change in HSV virulence.
LoxP: Recombination site for Cre recombinase. When Cre is present the region flanked by the two LoxP sites will be excised.
EGFP or LacZ reporter: EGFP or LacZ driven by CMV promoter. It enables infected cells to be identified easily based on reporter expression.
BACYAC backbone: Bacterial artificial chromosome (BAC) and yeast artificial chromosome (YAC) backbone. It allows the vector to be selectively propagated in E. coli using chloramphenicol resistance gene and Saccharomyces cerevisiae using His3 auxotrophic selection marker.
Our HSV BACYAC vectors cover HSV-1 wildtype strains. Additionally, we offer mutagenesis services (point mutations, deletions or insertions) by BAC recombineering on all our HSV vectors or on customer supplied vectors. While BACs harboring HSV genomes with deletions/mutations in genes essential for viral replication can be used for generating replication-defective HSV, BACs harboring HSV genomes with deletions/mutations in non-essential genes can be used for generating attenuated HSV.
HSV virus packaging
Price and turnaround Price Match
| Scale | Application | Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|
| Ultra-purified pilot | Cell culture & in vivo | >107 PFU/ml | 1 ml (10x100 ul) | $2,099 | 28-35 days |
| Ultra-purified medium | >108 PFU/ml | $3,099 |
Shipping and storage
Our HSV is stored in HBSS buffer and is shipped on dry ice. Upon receiving, it should be stored at -80°C for long term (stable for at least 6 months), or -20°C for use within one week. The shelf life for HSV is approximately one year. Please avoid repeated freeze-thaw cycles of HSV, as this can result in a large titer drop.
HSV amplicon vector cloning
VectorBuilder offers HSV-1 amplicon plasmids containing minimal viral sequences, namely the origin of replication (oriS) and packaging sequence (pac), plus one or more gene(s) of interest. Amplicon plasmids can be packaged into infectious but replication-defective viral particles in the presence of helper functions that can be provided in the form of a BAC carrying the entire HSV-1 genome except for the viral packaging signals.
The basic design of our HSV-1 amplicon plasmid is depicted in Figure 2 below:
Figure 2. Map of HSV-1 amplicon plasmid.
Promoter: The promoter that drives your gene of interest is placed here.
Kozak: Kozak consensus sequence. It is placed in front of the start codon of the ORF of interest because it is believed to facilitate translation initiation in eukaryotes.
ORF: The open reading frame of your gene of interest is placed here.
BGH pA: Bovine growth hormone polyadenylation. It facilitates transcriptional termination of the upstream ORF.
HSV-1 pac: Herpes simplex virus 1 packaging signal required for the packaging of viral DNA into virus.
Ampicillin: Ampicillin resistance gene. It allows the plasmid to be maintained by ampicillin selection in E. coli.
pUC ori: pUC origin of replication. Plasmids carrying this origin exist in high copy numbers in E. coli.
oriS: HSV-1 origin of DNA replication. It allows the plasmid to be amplified and packaged into HSV-1 particles as a concatemeric DNA.
IE4/5: HSV-1 ICP22 and ICP47 immediate early gene promoter. The activity of promoter is dependent on the transacting HSV-1 tegument protein VP16. It drives the ubiquitous expression of the downstream marker gene. It also works as a transcriptional regulatory region flanking oriS stimulating origin function.
Marker: A detectable gene (such as EGFP, LacZ). This allows cells transduced with the vector to be visualized.
SV40 early pA: Simian virus 40 early polyadenylation signal. It facilitates transcriptional termination of the upstream ORF.
Technical Information
HSV production and quality control (QC)
For HSV packaging (Figure 3), the BACYAC plasmid carrying the gene of interest (GOI) is transfected along with Cre plasmid into packaging cells. Nonfluorescent plaques are then selected to purify virus from which the BACYAC backbone is removed. Then, selected plaques are applied to new dishes to amplify HSV. For ultra-purified HSV, viral particles are further purified by sucrose density gradient centrifugation and concentrated.
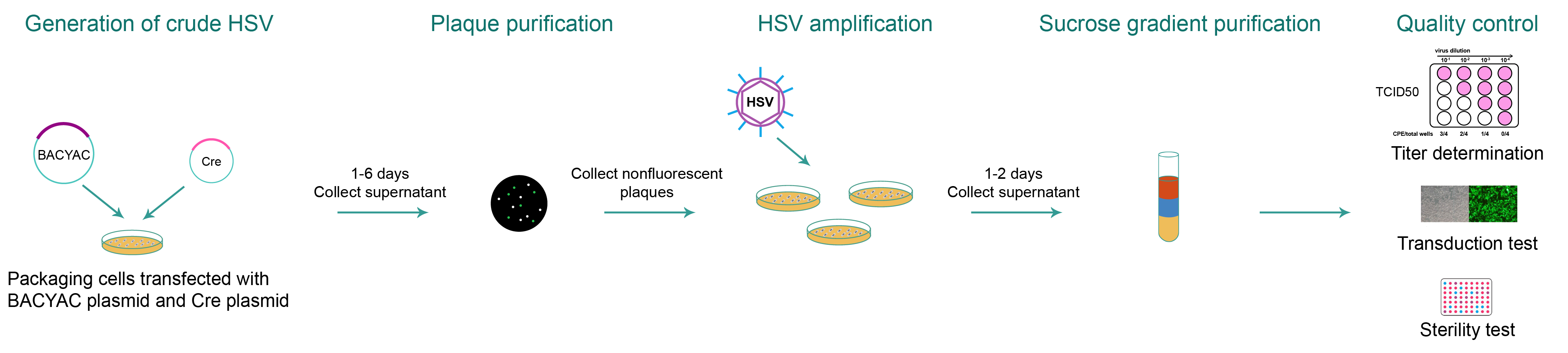
Figure 3. Typical workflow of HSV packaging from BACYAC vector with the BACYAC backbone removed.
For each recombinant HSV produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the HSV vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. If the HSV vector encodes a drug-selectable marker, we would perform transduction test followed by corresponding drug selection. Additionally, for ultra-purified HSV, we routinely perform endotoxin assay to check the endotoxin level.
Experimental validation
Our HSV vectors have been fully validated to be capable of producing live virus. Examples of the successful packaging of wildtype HSV viral particles using our BACYAC vector followed by their transduction into target cells is shown below in Figure 4 and Figure 5, respectively.
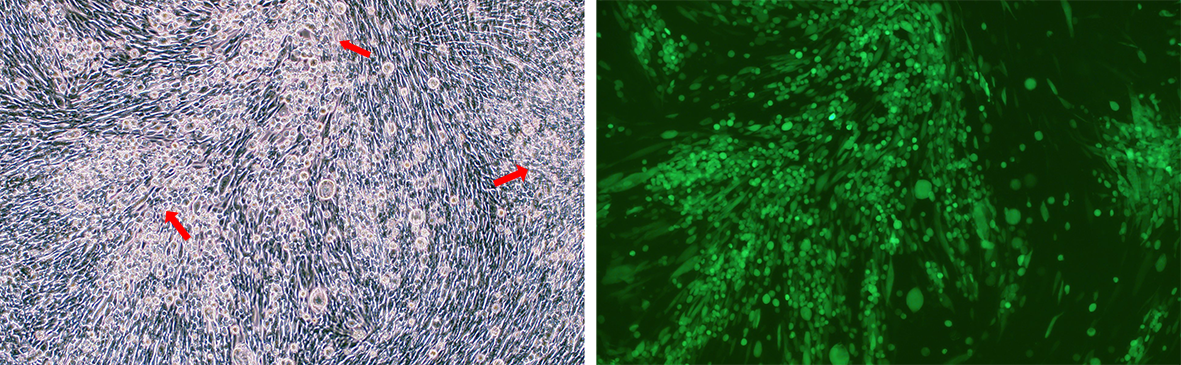
Figure 4. BHK21 cells were transfected with our BACYAC vector carrying the full genome sequence of wildtype HSV-1 (KOS strain) along with an EGFP reporter. Images were taken at 72 hours post-transfection. Signs of cytopathic effect (CPE), namely clumps of dying cells with round morphology and increased light refraction (indicated by red arrows), can be seen demonstrating the presence of live virus. Magnification: 100x.
Left: bright field. Right: EGFP.
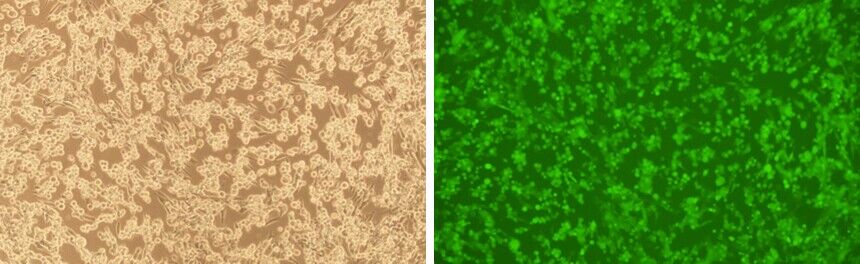
Figure 5. BHK21 cells were transduced with HSV particles produced from our BACYAC vector carrying the full genome sequence of wildtype HSV-1 (KOS strain) along with an EGFP reporter. Images were taken at 96 hours post-transduction. Magnification: 100x. Left: bright field. Right: EGFP.
Advantages of using HSV for gene therapy
HSV is an enveloped, double stranded DNA virus which offers several advantages, making HSV vectors attractive candidates for gene therapy. The major advantages offered by HSV vectors are discussed below:
Broad tropism: A wide range of dividing and nondividing cell types can be readily transduced by HSV vectors. Additionally, HSV is naturally neurotrophic and has the ability to achieve highly efficient gene delivery to neurons distal from the site of inoculation due to retrograde axonal transport. This makes HSV vectors ideal for treating certain neurological disorders.
Latent behavior: HSV has the ability to maintain itself in a latent state within sensory neurons indefinitely, without any detectable adverse effects. The ability to persist in a latent state allows HSV to evade the host immune system and persist within host cells for its lifetime. This offers the advantage of using HSV vectors for stably expressing gene(s) of interest in neurons for studies requiring long-term expression.
Minimal risk of insertional mutagenesis: When maintaining latent infection, HSV remains as an episome without integrating into the host genome, thereby minimizing the risk of insertional mutagenesis.
Ability to grow in tissue culture: HSV can be grown in tissue culture very easily and has been shown to establish latent infection in various animal models.
High viral titer: HSV vectors can be packaged into high titer virus which enables target cells to be transduced with high efficiency.
Large cargo capacity: HSV has a large genome size containing more than 80 genes, several of which are non-essential for replication. This provides the flexibility of deleting these non-essential genes and utilizing the available space for expressing large or multiple transgenes.
Commonly used HSV vectors
HSV vectors can be broadly classified into the following categories:
HSV BAC vectors
HSV BACs are generated by the insertion of a BAC backbone sequence into the HSV genome through homologous recombination in viral permissive eukaryotic cells. Presence of a selectable marker such as EGFP helps in the purification of recombinant viruses that have successfully incorporated the BAC sequence. Herpesvirus genomes have the tendency to circularize while replicating in the host cell nucleus producing replication intermediates which are then isolated for transforming E. coli. Presence of an antibiotic resistance gene in the BAC backbone facilitates the selection of bacterial cells carrying viral BACs. The final step involves the isolation of viral BAC DNA from the bacterial cells to validate its integrity by restriction digestion and sequencing analysis. Once validated, the HSV BACs are transfected into viral permissive eukaryotic host cells to generate live virus.
Since HSVs have a large genome size, using regular plasmid vectors for cloning HSVs is not feasible. BACs on the other hand are the preferred vectors for studying HSV biology due to their ability to carry large DNA sequences and slow replication rate. HSV BACs also help to confer stability to viral sequences when grown in E. coli as compared to propagating clinical isolates of the virus in permissive cells. Additionally, HSV BACs offer the flexibility to manipulate the viral genome very easily and accurately using common genetic engineering techniques to produce mutant viruses of interest.
Although HSV BACs offer several advantages as mentioned above, they do have a few limitations which should be taken into consideration. HSV BACs should be checked periodically for the presence of undesired mutations due to the unstable natural of repetitive sequences in the viral genome. Additionally, presence of the BAC backbone within the viral genome causes an increase in the viral genome size which has been shown to hinder viral growth. Therefore, the ideal approach for designing HSV BACs is to incorporate loxP or FRT sites flanking the BAC backbone which provide the flexibility to remove the BAC backbone by Cre- or Flp-based recombination, respectively.
HSV amplicon vectors
Amplicons are HSV-1 based plasmids containing one or more gene(s) of interest in addition to minimal viral sequences, which can be packaged into live HSV particles in the presence of helper functions supplied in trans either by HSV-1 helper virus or by cloned HSV-1 genome. Amplicons contain only two sequences of viral origin, an origin of replication known as oriS that allows viral replication in packaging cells and a packaging sequence known as pac that facilitates their packaging into live virus. HSV particles are produced by the replication of the amplicon genome via a rolling-circle like mechanism, similar to that used by wildtype HSV-1 leading to the generation of head-to-tail concatemers consisting of tandem repeats of the amplicon plasmid.
Amplicon vectors offer several advantages as gene transfer tools such as their large cargo carrying capacity up to 150 kb, the ability to clone them very easily, and their ability to transduce a wide range of cell types and their high transduction efficiency. Additionally, the absence of viral protein encoding sequences renders amplicon vectors completely non-toxic and non-pathogenic to transduced cells and organisms. The lack of viral genes in the amplicon vectors also helps to minimize their chances of reactivating or recombining with viral genomes that might reside in a latent stage within the host cells.
For a long time, the major limitation of amplicon-based gene delivery has been the generation of high-titer amplicon vector stocks that are free of the helper virus. Amplicon stocks contaminated with helper virus are not desirable for gene therapy applications as it could lead to potential cytotoxicity and inflammatory responses. The development of a helper virus free packaging system which utilizes a set of overlapping cosmids or a BAC vector containing the entire HSV-1 genome excluding just the viral packaging signal, for supplying the helper function has helped overcome this problem to a great extent. Although this method can lead to the generation of almost helper virus free amplicon stocks, it is limited by the inability to achieve high viral titers. An alternative approach for obtaining high-titer amplicon stocks with reduced contamination with helper viruses utilizes the Cre-lox technology to delete the packaging signal of the helper virus in the virus producing cells. However, since amplicon stocks produced using this method could still be contaminated with very low levels of the helper virus, that could still limit their use in certain gene therapy applications.
Replication-defective HSV vectors
Replication-defective HSV vectors are produced by the deletion or mutations of genes essential for viral replication. Replication-defective HSVs can grow only when the missing viral proteins are complemented in trans using engineered cell lines. Most replication-defective HSV vectors reported so far have been generated by the deletion of immediate-early (IE) genes encoding the infected cell proteins (ICPs), including ICP0, ICP4, ICP22, ICP27 and ICP47, in various combinations. The ICPs are expressed shortly after HSV enters host cells to initiate the transcription of a series of early (E) and late (L) viral genes that encode proteins essential for viral genome replication and assembly of the virion structure, respectively. Therefore, deletion of the IE genes leads to an inhibition of early and late viral gene expression.
The latest versions of replication-defective HSV vectors have been generated by the deletion of multiple IE genes which have led to these vectors being significantly less cytotoxic compared to their previous versions which were generated by the deletion of fewer IE genes. The deletion of multiple essential genes along with some non-essential genes in these vectors also increases their available cargo carrying capacity, thereby offering the advantage of using them to express multiple independent gene expression cassettes. This makes these vectors extremely suitable for gene therapy applications requiring the simultaneous expression of multiple transgenes.
The primary disadvantage of replication-defective HSV vectors is the cytotoxicity observed with vectors generated by the deletion of single IE genes. Although problems with cytotoxicity can be significantly reduced by developing vectors that are deleted for all five IE genes, such vectors have been shown to exhibit poor growth in cell culture and reduced levels of transgene expression. Therefore, the deletion of essential genes in the right combination is critical for achieving the optimal performance of replication-defective HSV vectors. The other disadvantage of replication-defective HSV vectors is that growing these vectors strictly relies on the availability of complementing cell lines which can supply the deleted viral proteins in trans.
Attenuated HSV vectors
Attenuated HSV vectors (also known as conditional replicating HSV vectors) are generated by the deletion or mutation of genes that are not essential for the replication of virus in vitro, such as genes involved in various aspects of in vivo virus-host interactions. Therefore, attenuated HSVs are capable of replicating in cell culture but are incapable of replicating in vivo under specific conditions. Using this approach, several attenuated HSV vectors incapable of replicating in normal cells but capable of selectively replicating in tumor cells have been developed for being used as oncolytic agents.
Attenuated HSV vectors offer the advantage of achieving significantly more efficient gene transfer as compared to non-replicating HSV vectors, due to their ability to replicate and spread infection easily. Additionally, using attenuated HSV vectors as viral vaccines provide the opportunity for the host’s immune system to be exposed to all the viral antigens, thus leading to the stimulation of both cell-mediated and humoral immunity. Moreover, such vectors can be grown in cell culture with relative ease and therefore, present a cost-effective approach for vaccine development.
The major limitation associated with attenuated HSV vectors are several potential safety concerns which might arise due to their ability to replicate. Risks associated with attenuated HSV vectors include mutation or recombination of the replicating virus into a more pathogenic strain in vivo, chances of the virus or its mutant spreading from the patient to others, and chances of the virus or its mutant being pathogenic to the patient or a developing fetus. Another disadvantage of attenuated vectors is the possibility of over attenuating the virus which in turn might cause a significant decrease in its effectiveness. Therefore, careful selection of genes to be deleted or mutated is crucial for the generation of an attenuated vector with desired functionality.
Major applications of HSV vectors
Recombinant HSV vectors are used for a variety of gene therapy applications. Below are some of the major research areas where HSV vectors have been and are being extensively used:
HSV vectors as oncolytic agents
Several factors make HSV-1 vectors attractive candidates for oncolytic virotherapy. HSV-1 is highly infectious, completing its entire replication cycle in 10 hours to release thousands of progeny virions, which is much shorter compared to the time taken by other common viruses such as adenovirus. HSV-1 virions can spread from one cell to another through cell junctions in addition to extracellular spreading, allowing highly efficient virus spread within solid tumors. Additionally, HSV-1 has been shown to effectively infect a variety of laboratory animals, thus making it highly suitable for in vivo preclinical studies.
Attenuated HSV vectors designed to selectively replicate in and kill cancer cells while being unable to grow in normal cells have been widely used as oncolytic viruses. Such vectors can be generated by deleting genes that are indispensable for viral replication in normal cells but are not essential for tumor cells. The anti-tumor effects of such vectors can be further enhanced by modifying them to carry genes that are either anti-tumorigenic or can activate chemotherapeutic agents. Talimogene Laherparepvec (TVEC), an FDA approved drug that is currently used for treating melanoma is an example of an attenuated HSV-1 vector with oncolytic potential. TVEC was derived from HSV-1 by deleting viral genes ICP34.5 and ICP47 that leads to the inhibition of viral replication in normal cells and activation of the immune system, respectively. The oncolytic potential of TVEC is further augmented by the overexpression of the gene encoding human granulocyte-macrophage colony-stimulating factor (GM-CSF), which further stimulates the immune system by enhancing the migration and maturation of dendritic cells.
In addition to attenuated vectors, replication-defective HSV vectors have also been widely used for treating a variety of cancers. These approaches primarily focused on utilizing replication-defective HSVs for driving the expression of suicide genes such as thymidine kinase (TK) or genes with therapeutic potential such as TNF-α within tumor cells, either by themselves or in combination.
HSV vectors as vaccines
HSV vectors offer several advantages for use as viral vaccines against viral and bacterial pathogens, including: 1) their ability to induce strong immune reactions in host organisms in response to inoculation via various routes; 2) their ability to persist as episomes in the host cell nucleus without integrating into the host genome; and 3) the natural presence of the TK gene in the HSV genome which has the ability to convert non-toxic drugs such as ganciclovir into cytotoxic metabolites and therefore can be used to kill cells infected with the virus in the event of any undesired effects. Studies utilizing HSV-BACs, amplicon vectors, replication-defective HSV vectors and attenuated HSV have all shown promising results when used as viral vaccines against a variety of pathogens.
Attenuated HSV vectors are particularly suitable for the development of anti-HSV vaccines since they provide the opportunity for the host’s immune system to be exposed to all the viral antigens, thus leading to the stimulation of both cell-mediated and humoral immunity. Additionally, attenuated live vaccines have been shown to provide more long-term and effective protection as compared to inactive vaccines or vaccine vectors expressing only specific subunits of the viral genome. Given that a significant percentage of the world population is infected with HSV-1 and HSV-2 and currently no FDA approved vaccines are available to treat either of them, the urgency to develop anti-HSV vaccines is high. VC2, a live attenuated HSV-1 vector developed by partial deletions in the viral glycoprotein gK and the membrane protein UL20 has shown great potential as a vaccine candidate when tested in animal models of HSV-1 and HSV-2 infection.
Replication-defective HSV mutants with deletions in genes essential for viral replication and expressing specific foreign antigens have also been shown to induce persistent and potent immune responses when administered as vaccines in various animal models of viral and intracellular bacterial infections. Additionally, amplicon vectors have been widely applied as therapeutic vaccines against cancer, microbial infections and neurological diseases in a number of pre-clinical studies using mouse models. However further studies are needed to evaluate their immunogenic potential in other animal models, including those with pre-existing HSV immunity.
HSV vectors as gene delivery vehicles
HSV amplicon vectors are particularly well-suited as gene delivery vehicles for neurons due to their natural neurotropism, large cargo carrying capacity (<150 kb), low toxicity and ability to remain as episomes within the target cells. Therefore, they have been widely used for research in the following areas: 1) neurodegenerative diseases such as Alzheimer’s and Parkinson’s; 2) neuropsychiatric disorders including depression and addiction; and 3) treatment of diseases requiring the delivery of therapeutic genes into the nervous system such as acute ischemic stroke. Additionally, due to their ability to infect a wide variety of dividing cell lines and the relative ease of infecting cells with HSV amplicon vectors compared to plasmid transfection, amplicon vectors are highly suitable for in vitro pharmacological research.
Replication-defective HSV vectors are also used as gene delivery vehicles for gene therapy applications due to their significantly reduced cytotoxicity achieved by the deletion of genes essential for viral replications. Deletion of multiple genes also increases the cargo capacity for these vectors, enabling them to carry multiple independent gene expression cassettes which is typically a requirement for vectors used in multiplex gene therapy. Therefore, replication-defective vectors have been widely used for gene therapy studies in animal models of chronic pain, Parkinson’s disease, spinal cord injury and lysosomal storage disorders. NP2, a replication-defective HSV vector expressing human preproenkephalin (PENK) has shown promising results in a phase I clinical trial for treating pain.
How to Order
Resources
FAQ
HSV is naturally neurotropic, offers a large cargo capacity of up to 150 kb when used as amplicons and has the ability to maintain itself in a latent state for the lifetime of host cells, which renders it with some unique advantages over other commonly used viral vectors such as lentivirus, AAV or adenovirus. Additionally, HSV has a broad host cell range, maintains itself as an episome instead of integrating into the host genome, can be easily grown in cell culture, and can be packaged into high titer virus.
VectorBuilder offers HSV BACYAC vectors and amplicon vectors, both of which can be used for producing live virus and we have carried our extensive testing to validate this. HSV BACYACs can be transfected into viral permissive eukaryotic host cell lines such as BHK21 for producing live virus. For amplicon vectors, co-transfection of the amplicon plasmid along with a BAC containing packaging defective HSV-1 genome into appropriate packaging cells leads to the generation of live virus. Figure 4 shows the successful packaging of wildtype HSV-1 viral particles using our vector.
HSV vectors can typically be handled in a biosafety level-2 (BSL-2) containment. However, biosafety policies can vary considerably from one institution to another. Therefore, it is the responsibility of the researchers to handle all viral vectors following appropriate biosafety guidelines that apply for their institution.




