IVT RNA and LNP Manufacturing

VectorBuilder offers a full range of CRO and CDMO services for in vitro transcription (IVT) mRNA manufacturing and lipid nanoparticle (LNP) therapeutic development. Relying on our revolutionary vector design platform and extensive experience, we can provide optimal in vitro transcription vector designs, large-scale IVT mRNA manufacturing, and LNP encapsulation followed by thorough quality control tailored to a wide range of research and clinical needs. We offer several grades that cover different downstream needs including drug discovery research and pre-clinical studies.
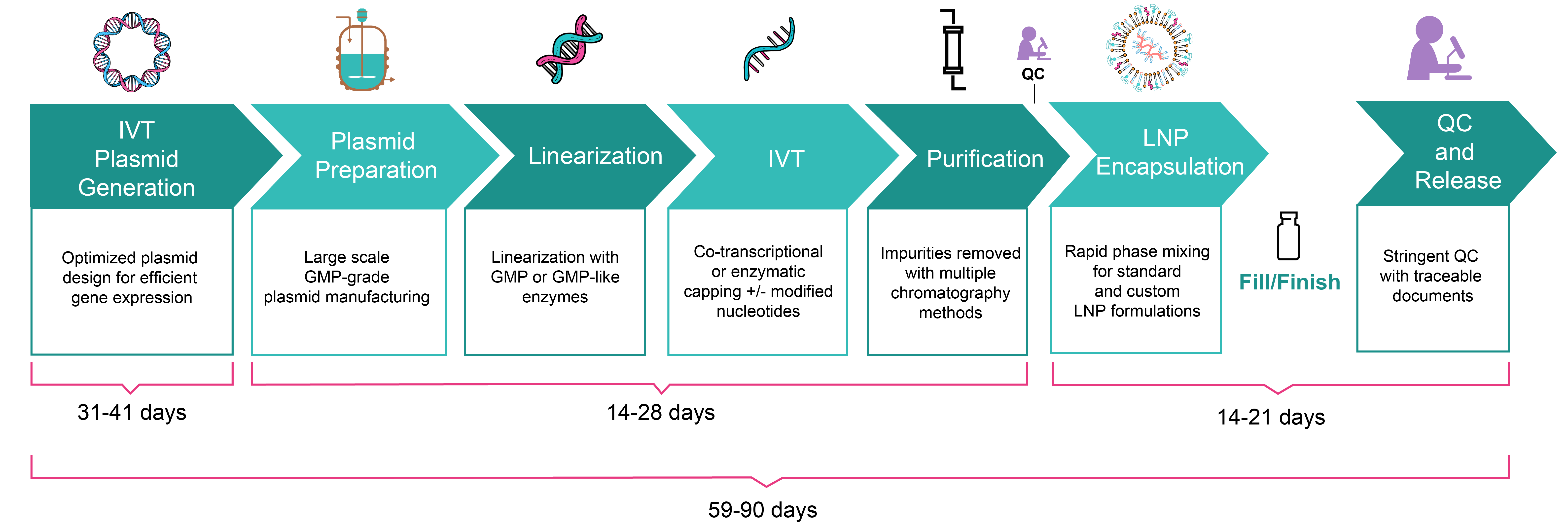
Workflow for Production of IVT mRNA
Grades of IVT mRNA Offered
-
Research-grade mRNA
Research-grade mRNA is intended for basic research and drug discovery studies. It is made under standard laboratory conditions with stringent QC to ensure high quality suitable for all downstream research needs.
Learn more about our research-grade mRNA -
GMP-like mRNA
GMP-like mRNA is intended for pre-clinical studies such as animal testing of drug safety and metabolism. It is produced in a manner that adopts key features of GMP guidelines, including comparable production process and similar quality attributes. Production is performed in segregated production suites with document control and traceability. GMP-like grade can thus be viewed as a small-scale mimic of the final GMP product, but with much lower cost and faster timeline. Where appropriate, GMP-like mRNA can be produced under and RNase-free fermentation and purification conditions. A certificate of analysis (COA) is provided at the product release. TSE/BSE statement is available upon request.
-
GMP-grade mRNA Coming soon
GMP-grade mRNA is produced in our certified GMP suite with strict adherence to GMP guidelines. A comprehensive quality assurance system is implemented throughout the production process. A wide range of in-process and release QC assays are performed to ensure that the mRNA meets or exceeds the desired quality and safety standards. A batch release report fully documenting the production process and a COA are provided at product release. Other documentation is available upon request.
Comparison of different grades of IVT mRNA
| Research-grade | GMP-like | |
|---|---|---|
| Applications | Basic research, drug discovery, and preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism |
| Production scales | mRNA: 0.1-10 mg LNP: 0.1-3 mg |
mRNA: 0.01-20 g LNP: 3-20 mg |
| Turnaround time | 49-71 days ‧ Vector design & cloning: 26-36 days ‧ Plasmid production & linearization: 14-21 days ‧ IVT mRNA production: 14-21 days ‧ LNP encapsulation: 9-14 days |
59-90 days ‧ Vector design & cloning: 31-41 days ‧ Plasmid production & linearization: 14-28 days ‧ IVT mRNA production: 14-28 days ‧ LNP encapsulation: 14-21 days |
| Quality system | ISO9001 | ISO9001 while adopting key features of GMP system |
| Production facility | In parallel production in shared laboratory space | Productions done in segregated suites |
| Document control and traceability | No | Yes |
| QC and release | Standard QC | Performed on a case-by-case basis depending on individual project needs (see below) |
| Aseptic fill/finish | N/A | Available upon request |
| Storage of retention sample | Available upon request | Available upon request |
| Other deliverable | COA | 1. COA 2. Manufacturing summary 3. TSE/BSE statement upon request |
Lipid Nanoparticle Encapsulation
VectorBuilder has a full suite of instruments for lipid nanoparticle mixing and manufacturing followed by thorough quality control. Our production suite can accommodate custom lipid formulations and perform microfluidic mixing, dilution, buffer exchange, sterile filtration, and fill/finish using state-of-the-art equipment.
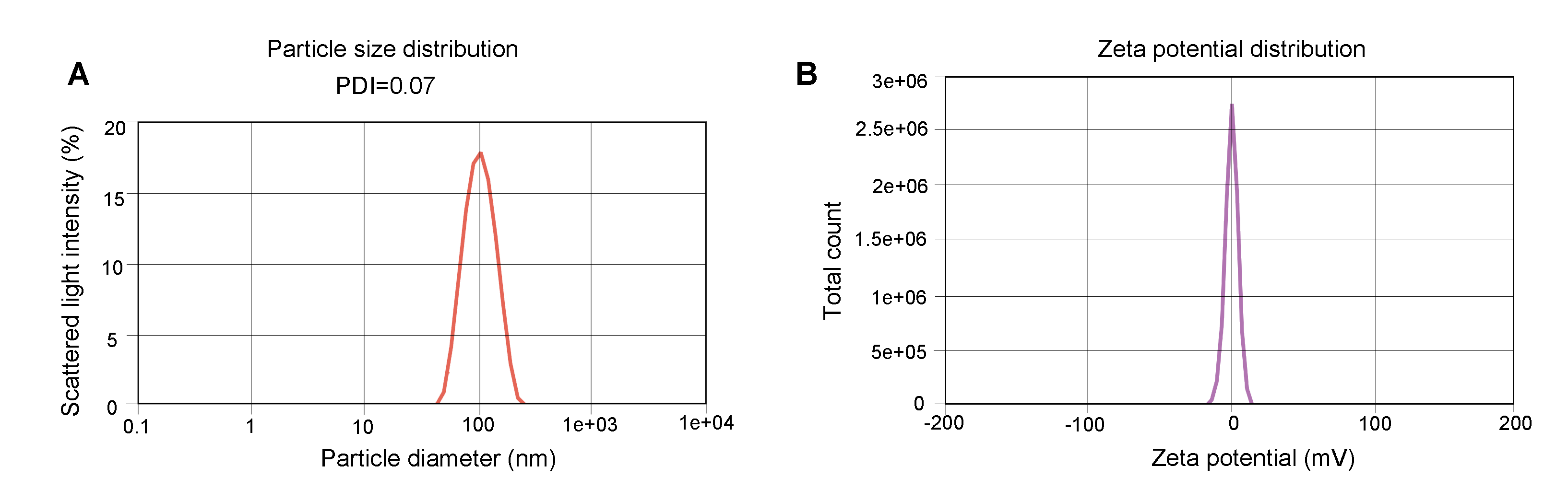
Figure 1. Representative QC results of LNP-mRNA.
(A) Particle size was determined by dynamic light scattering (DLS) which measures the intensity differences of fluctuated light due to motion of particles. The polydispersity index (PDI) reflects the heterogeneity of a sample on particle size. (B) Zeta potential reflects the stability of LNP. The Zeta potential of the sample is between -1.872 mV and +1.872 mV.
Quality Control Assays
| Product | Attribute | QC Assay |
|---|---|---|
| IVT DNA template | Concentration | Spectrometry |
| Identity | Gel electrophoresis, Sanger sequencing | |
| Linearization | Capillary gel electrophoresis | |
| Residual host E. coli DNA | qPCR | |
| mRNA | Concentration | UV-Vis spectrometry |
| Identity | Capillary gel electrophoresis, reverse transcription followed by Sanger sequencing | |
| Capping efficiency | LC-MS, Capillary gel electrophoresis | |
| PolyA tail integrity | LC-MS, Capillary gel electrophoresis | |
| Residual protein | NanoOrange assay | |
| Residual plasmid | qPCR | |
| dsRNA | Dot blot | |
| Endotoxin | Kinetic chromogenic assay (KCA) | |
| LNP | Endotoxin | Kinetic chromogenic assay (KCA) |
| Encapsulation efficiency | RiboGreen assay | |
| Diameter, PDI, and Zeta potential | Zetasizer |
Additional Offerings
In addition to mRNA, VectorBuilder offers IVT Cas9 mRNA and sgRNA for CRISPR gene editing as well as self-amplifying mRNA (saRNA). LNP formulations can be used to encapsulate IVT mRNA molecules, siRNA, and plasmid DNA allowing for multiple methods of gene delivery.



