Vesicular Stomatitis Virus (VSV) Packaging
Recombinant vesicular stomatitis virus (VSV) vectors are widely used for a variety of applications including studying mechanisms of viral entry into host cells, identification of cellular receptors utilized by viruses for cell entry, screening of viral entry inhibitors and vaccine development research. VSV vectors can be efficiently pseudotyped with envelope glycoproteins derived from heterologous viruses, such as envelope proteins of viruses requiring high-level containment, thereby making them highly suitable for studying the entry mechanisms of such high-risk viruses.
VectorBuilder has developed a series of proprietary technologies and reagents that have greatly improved recombinant VSV production protocols in terms of titer, purity, viability and consistency, especially for the VSV vector systems used in our vector cloning services.
Types of VSV offered
- VSV pseudotyped with VSV G protein
- VSV pseudotyped with coronavirus S protein and its variants
- VSV pseudotyped with any other envelope proteins as requested
- Bald VSV lacking viral envelope protein (can be used as negative control)
Service Details
VectorBuilder has developed a cloning strategy that utilizes two different backbones. Both contain all of the active genes in the VSV genome, except the viral fusion G protein. One backbone can be used to clone your GOI between the VSV-M and VSV-L genes (Figure 1a), and the other has the addition of reporters upstream of the VSV-N gene (Figure 1b). If you require other alterations to the backbone, please contact us and we’ll be happy to help.
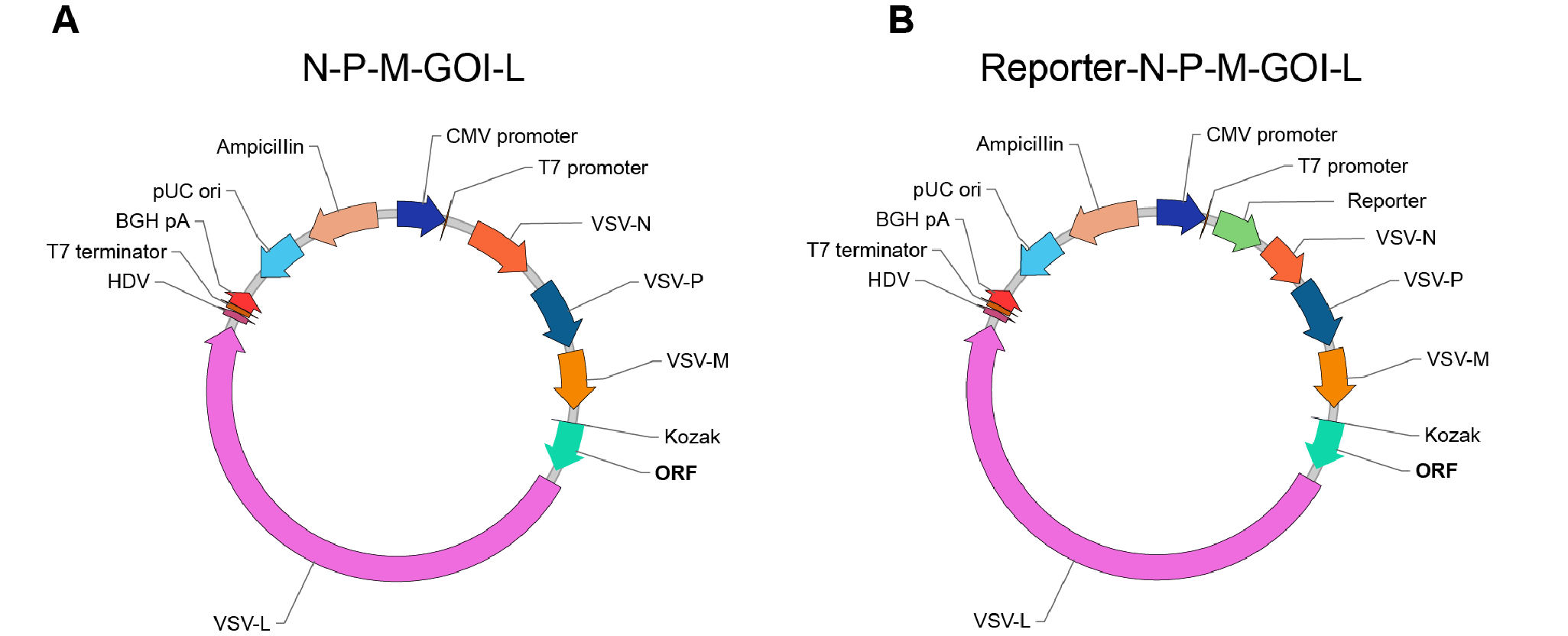
Figure 1. VSV backbones containing the N-P-M-L genes from the VSV genome.
Price and turnaround Price Match
| Scale | Application | Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|
| VSV pseudotyped with VSV G | |||||
| Medium | Cell culture | >107 PFU/ml | 1 ml (10x100 ul) | $1,099 | 21-35 days |
| Large | >108 PFU/ml | $1,399 | |||
| Ultra-purified medium | Cell culture & in vivo | >108 PFU/ml | 500 ul (5x100 ul) | $1,699 | |
| Ultra-purified large | 1 ml (10x100 ul) | $2,699 | |||
| VSV pseudotyped with SARS-CoV-2 S protein and its variants (Luc or EGFP as transgene) | |||||
| Medium | Cell culture | >107 PFU/ml | 1 ml (10x100 ul) | $2,199 | 21-35 days |
| Large | >108 PFU/ml | $2,799 | |||
| Ultra-purified medium | Cell culture & in vivo | >108 PFU/ml | 500 ul (5x100 ul) | $3,399 | |
| Ultra-purified large | 1 ml (10x100 ul) | $5,399 | |||
| VSV pseudotyped with SARS-CoV-2 S protein and its variants (other transgenes) | |||||
| Medium | Cell culture | >107 PFU/ml | 1 ml (10x100 ul) | $2,199 | 21-35 days |
| Large | >108 PFU/ml | $2,799 | |||
| Ultra-purified medium | Cell culture & in vivo | >108 PFU/ml | 500 ul (5x100 ul) | $3,399 | |
| Ultra-purified large | 1 ml (10x100 ul) | $5,399 | |||
PFU = Plaque forming units
Deliverables
| For non-ultra-purified scales | For ultra-purified scales |
|---|---|
| Your custom VSV | Your custom VSV |
|
Free: control virus (VSV pseudotyped with VSV G)
|
Add-on purchase (optional): ultra-purified control virus (VSV pseudotyped with VSV G)
|
Control virus
The control VSV is designed to match the biological application of the custom virus and to be used for testing VSV transduction. For example, if the custom virus overexpresses a gene, then the control virus provided will be EGFP control VSV (VSV overexpressing EGFP). Detailed information on the control virus is shown below:
| Vector System | Control Virus Vector Name | Control Virus Vector ID |
|---|---|---|
| VSV gene expression system | pVSV[Exp]-EGFP | VB010000-9315gcp |
Shipping and storage
Our VSV is stored in HBSS buffer and is shipped on dry ice. Upon receiving, it should be stored at -80°C for long term (stable for at least 6 months), or -20°C for use within one week. The shelf life for VSV is approximately one year. Please avoid repeated freeze-thaw cycles of VSV, as this can result in a large titer drop.
Technical Information
VSV production and quality control (QC)
For VSV packaging (Figure 2), the VSV-ΔG plasmid lacking the glycoprotein (G) gene and carrying the gene of interest (GOI), along with helper plasmids containing VSV genes G, N, P, and L are co-transfected into packaging cells together with a T7 RNA Polymerase helper plasmid. The viral proteins produced from the packaging plasmids are required for assembly of viral particles containing the viral genome on the transfer plasmid. Utilizing this plasmid over conventional vaccinia virus-facilitated packaging decreases turnaround time, increases purity (no helper virus residue), and increases packaging efficiency. Following generation of G-complemented VSV, the supernatant collected from packaging cells is then applied to a new dish of cells transfected with a plasmid expressing envelope proteins to generate and amplify pseudotyped VSV stocks. For ultra-purified VSV (in vivo grade), viral particles are further purified by sucrose density gradient centrifugation and concentrated. We use plaque assay to measure VSV titer. The workflow below is a general guide, but if you would like further information, particularly about packaging VSV with alternative pseudotypes, please contact us.
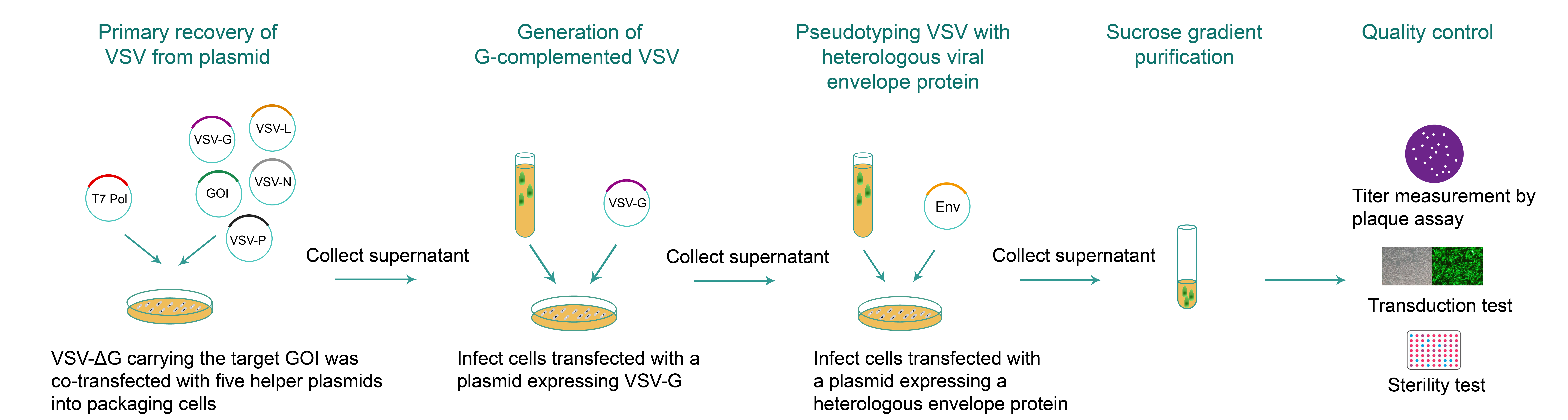
Figure 2. Typical workflow of VSV packaging.
For each recombinant VSV produced by VectorBuilder, quality control includes titer measurement, sterility testing for bacteria and fungi, and mycoplasma detection. If the VSV-delta G vector encodes a fluorescent protein, we would perform transduction test to detect corresponding fluorescence. If the VSV-delta G vector encodes a drug-selectable marker, we would perform transduction test followed by corresponding drug selection. Additionally, for ultra-purified VSV, we routinely perform endotoxin assay to check the endotoxin level. To include endotoxin results in your COA, an extra cost is required. Additional QC services can be provided upon request.
Experimental validation
We have developed a number of proprietary techniques to optimize our VSV packaging protocol. As a result, our optimized virus exhibits much higher transduction efficiency (Figure 3) as compared to published protocols.
Before transduction
After transduction
After transduction
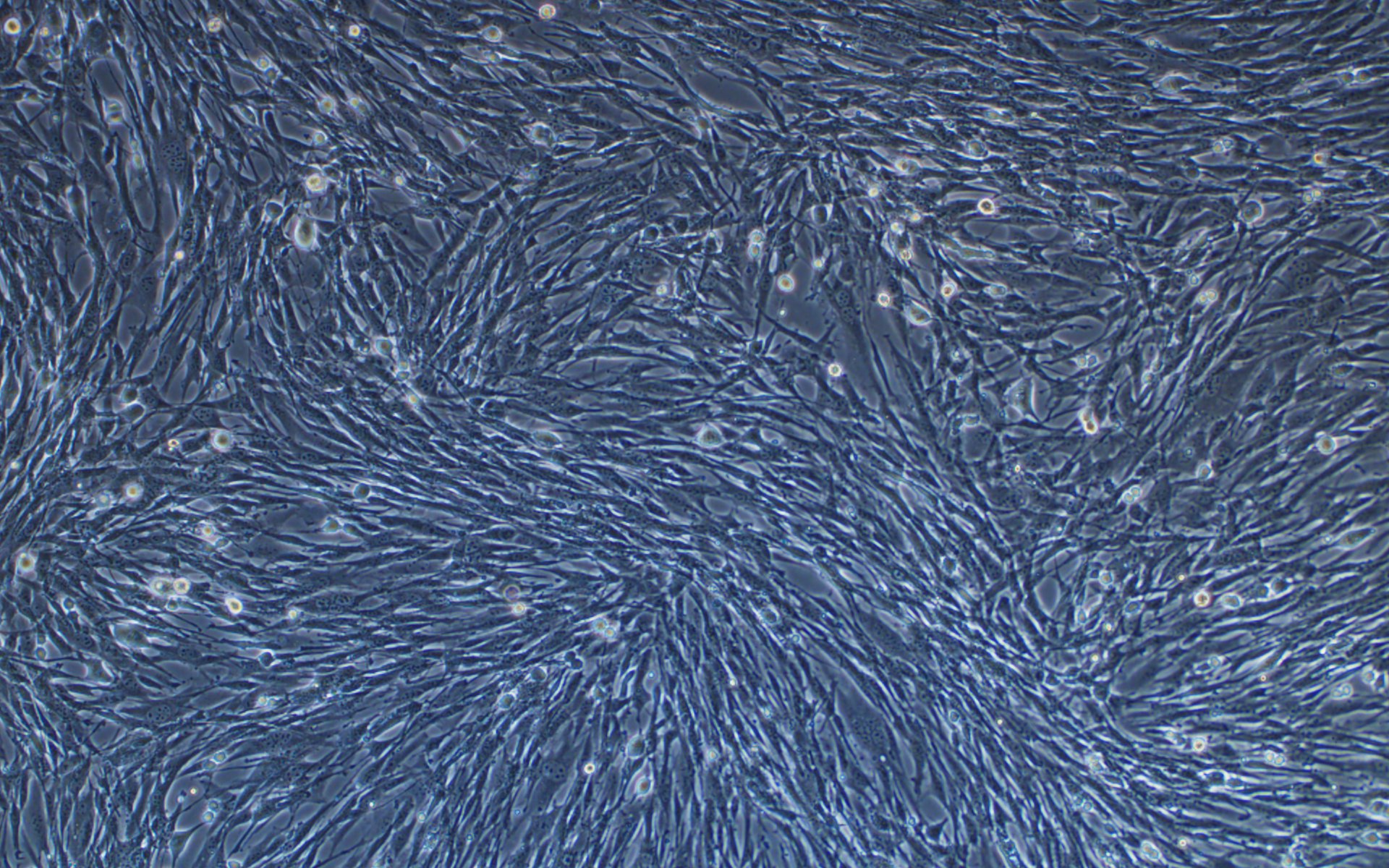

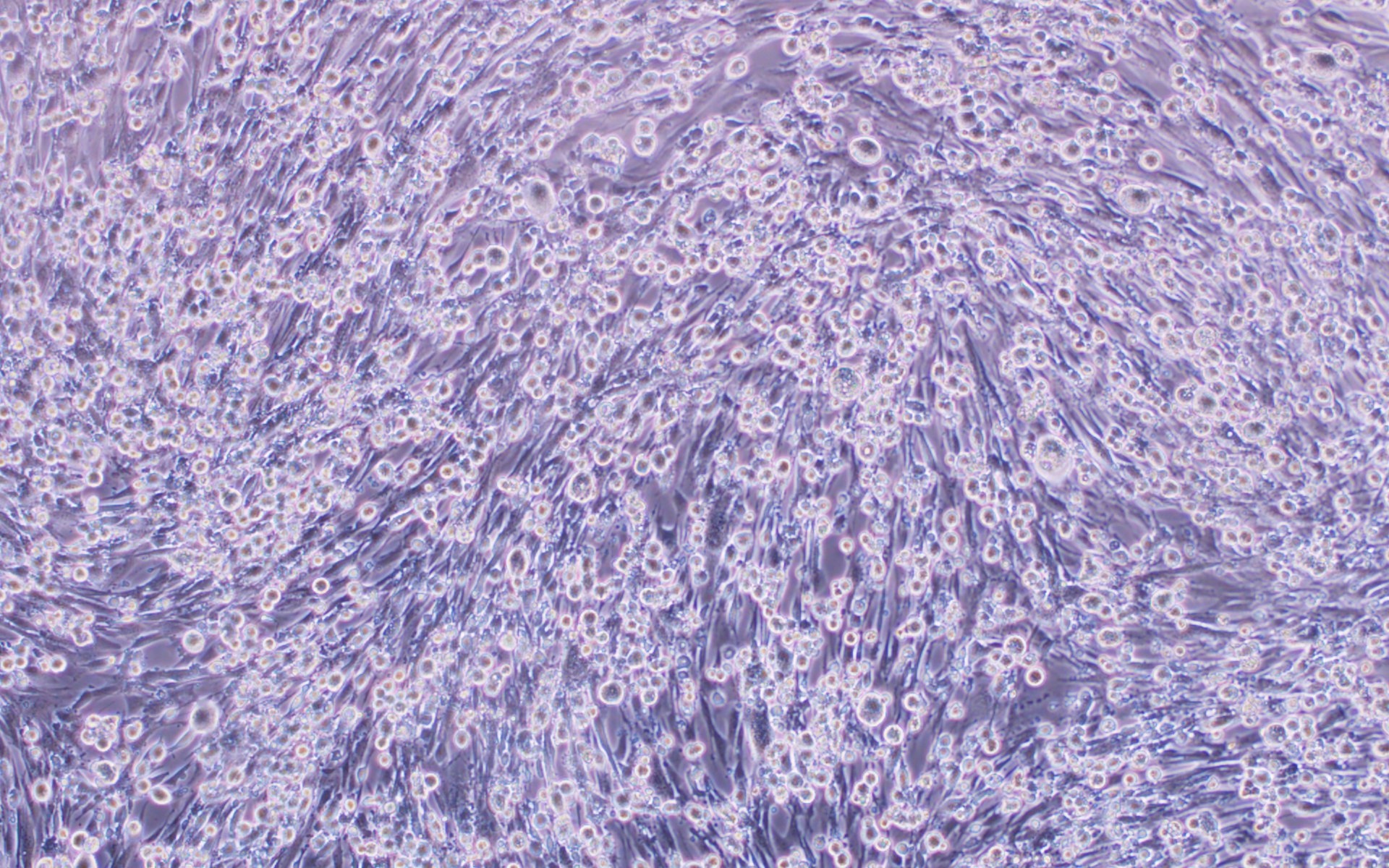
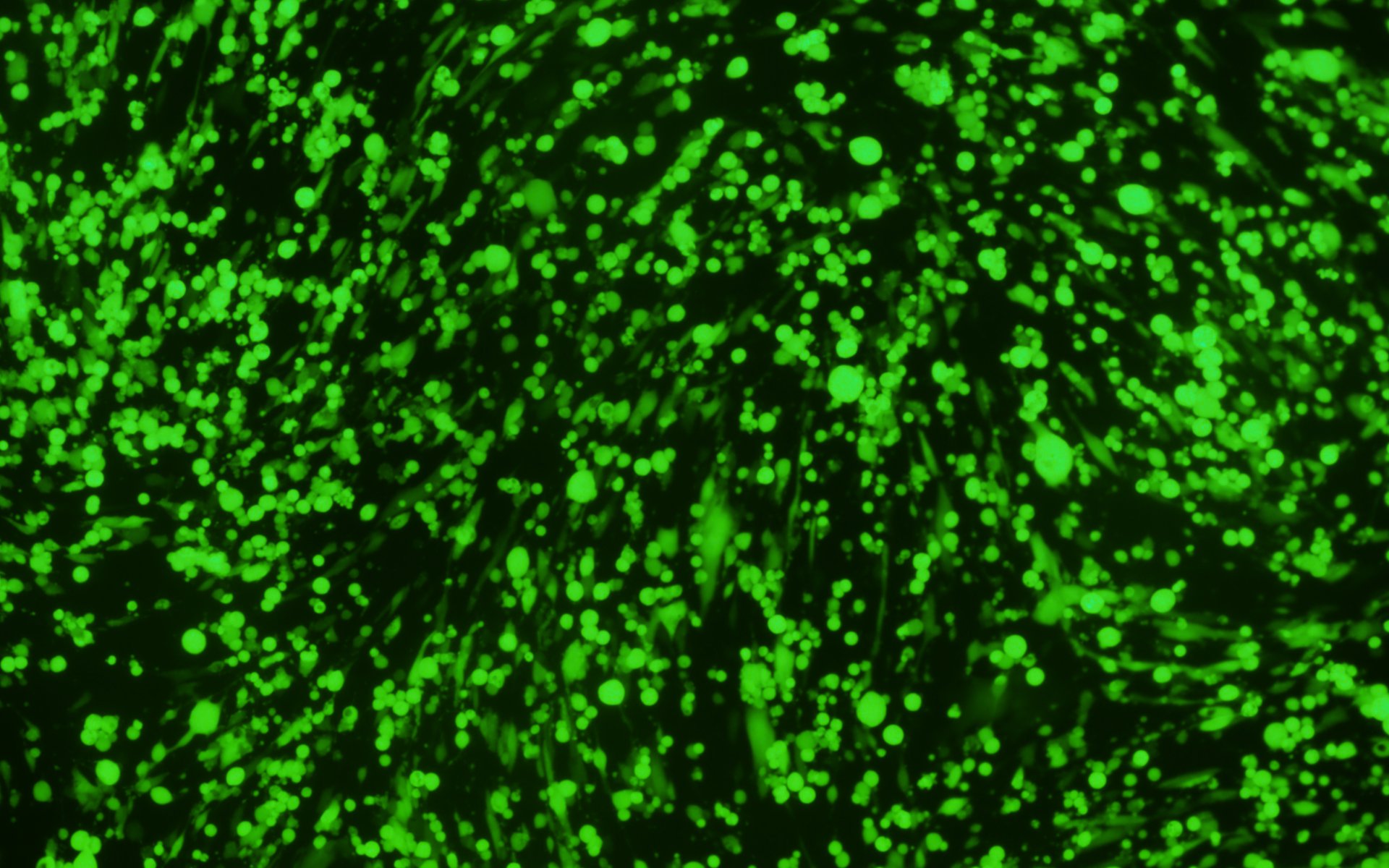
Figure 3. VSV pseudotyped with VSV G protein was used for transducing BHK-21 cells. Images were taken before transduction and at 24 hours post-transduction. Magnification: 100x. Left: bright field. Right: GFP.
Additionally, we have carried out extensive experimental validation of our VSV pseudotyping protocols with SARS-CoV-2 S protein and its D614G mutant. The results showed that SARS-CoV-2 S protein and its D614G mutant pseudotyped VSV could efficiently transduce BHK-21 cells expressing high levels of the human ACE2 receptor, but not BHK-21 cells in which ACE2 expression is absent or low, using a fluorescent reporter (Figure 4). We, therefore, recommend using our ACE2-expressing cell lines that have been optimized for transduction with SARS-CoV-2 S protein or VSV G pseudotyped virus.
S-pseudotyped VSV D614G S-pseudotyped VSV
BHK-21 cell line BHK-21-ACE2 cell line
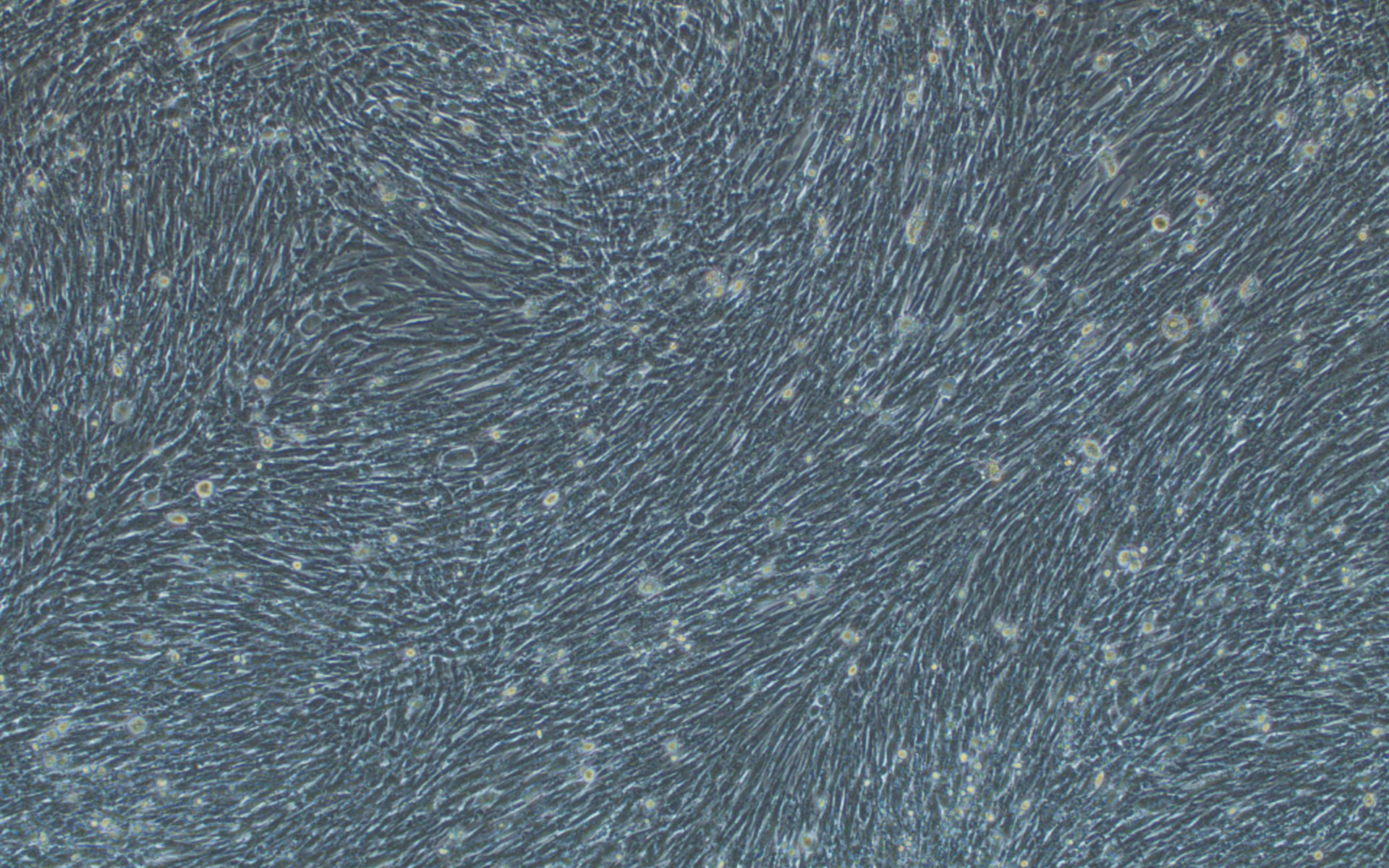

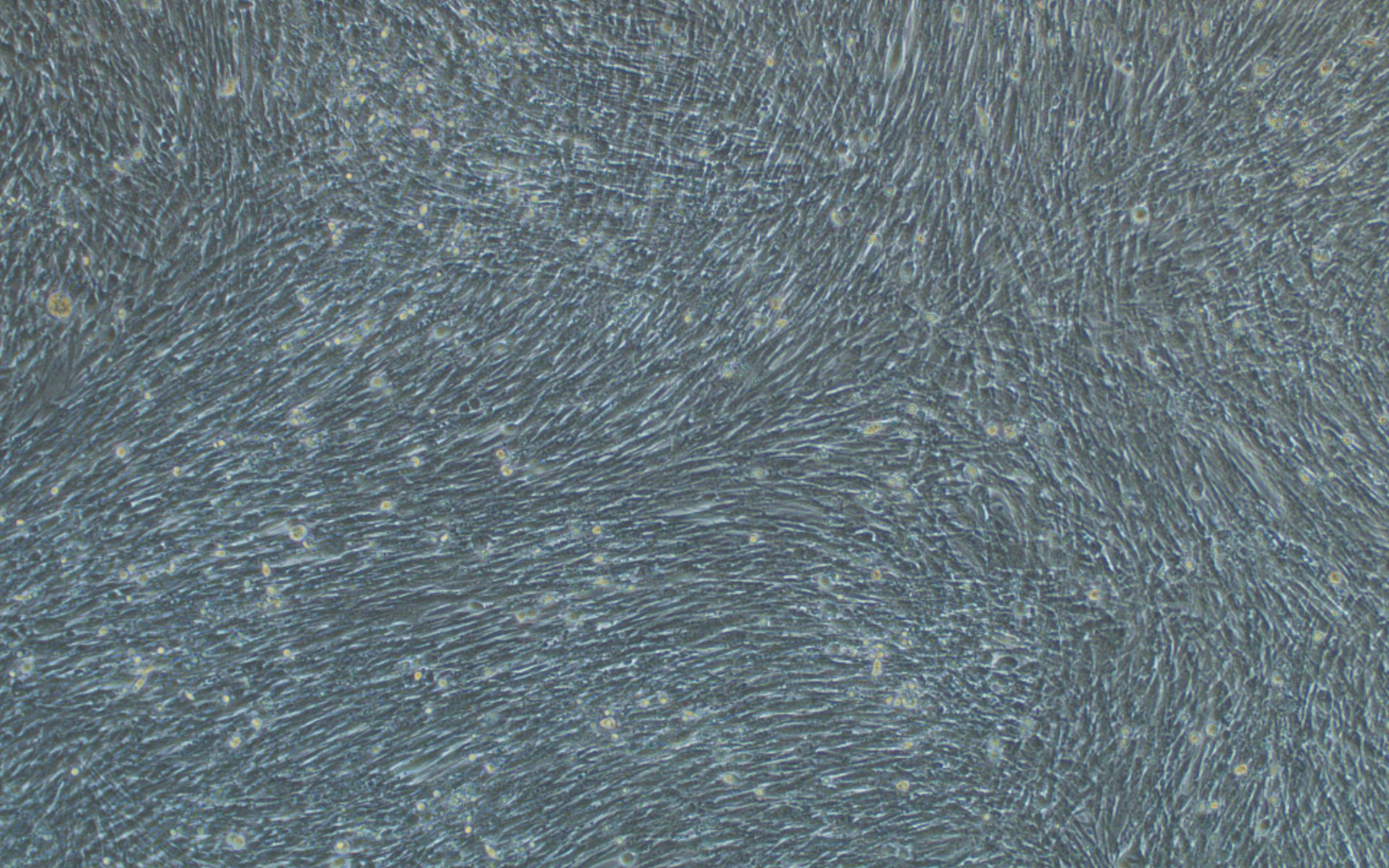

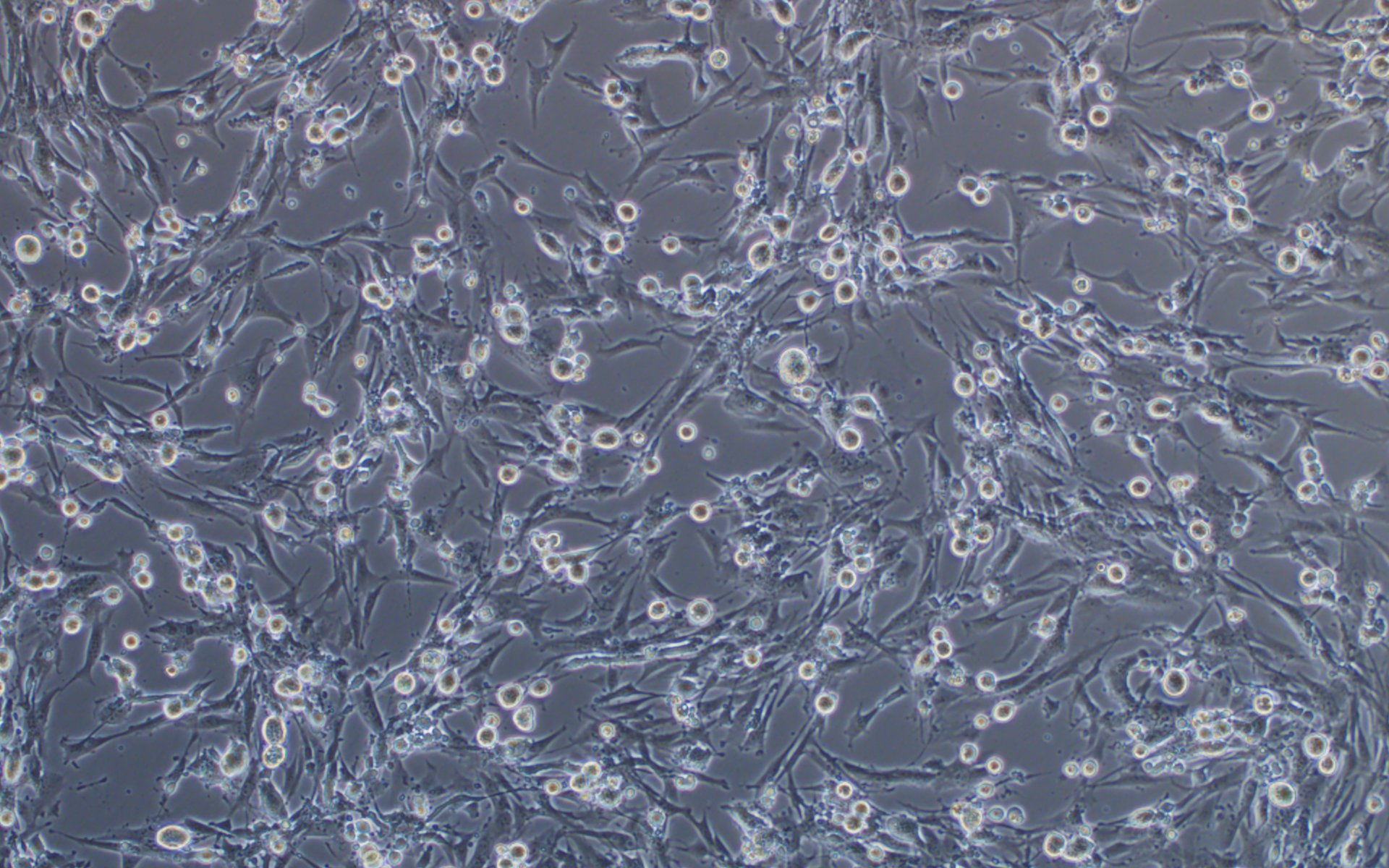
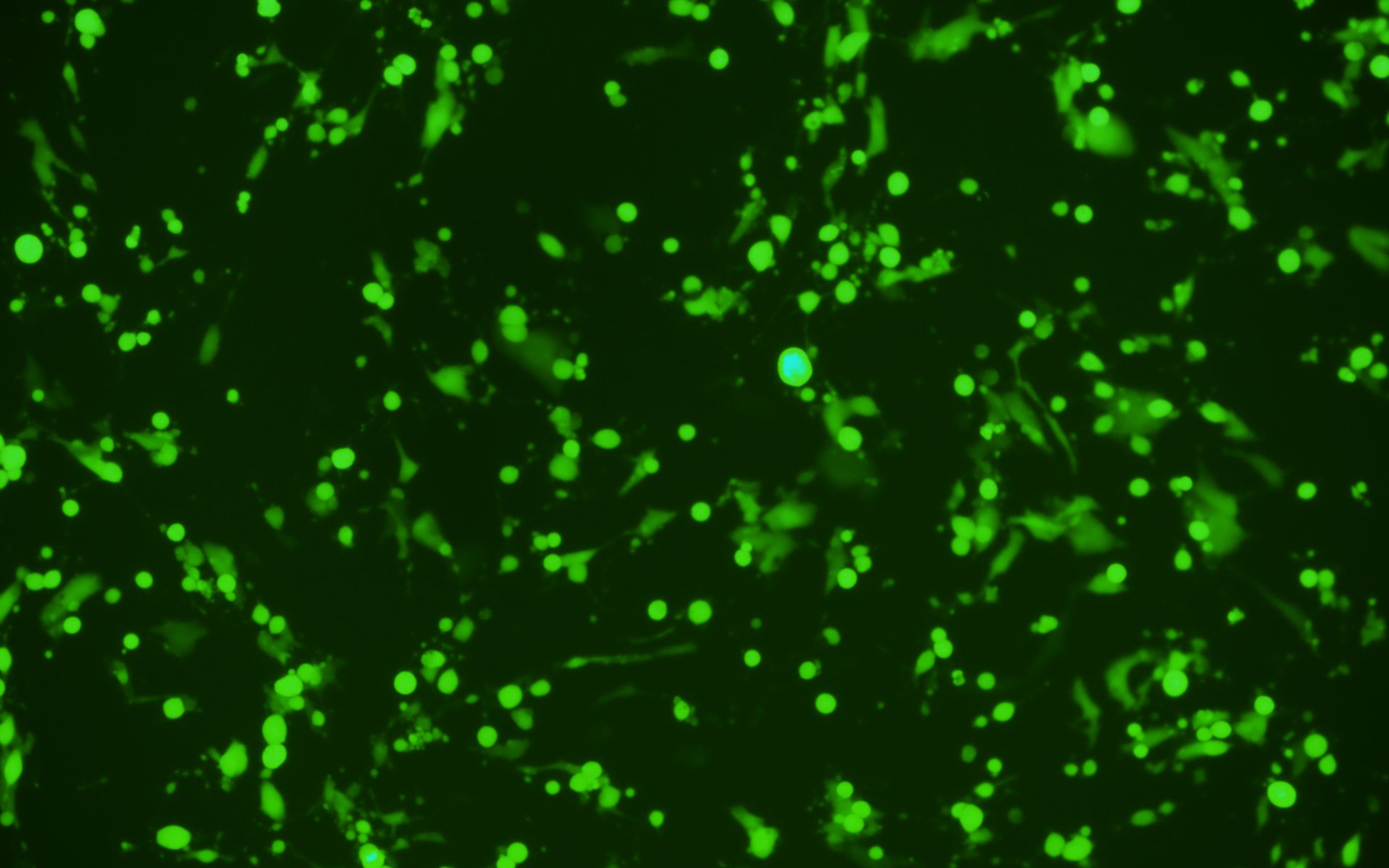
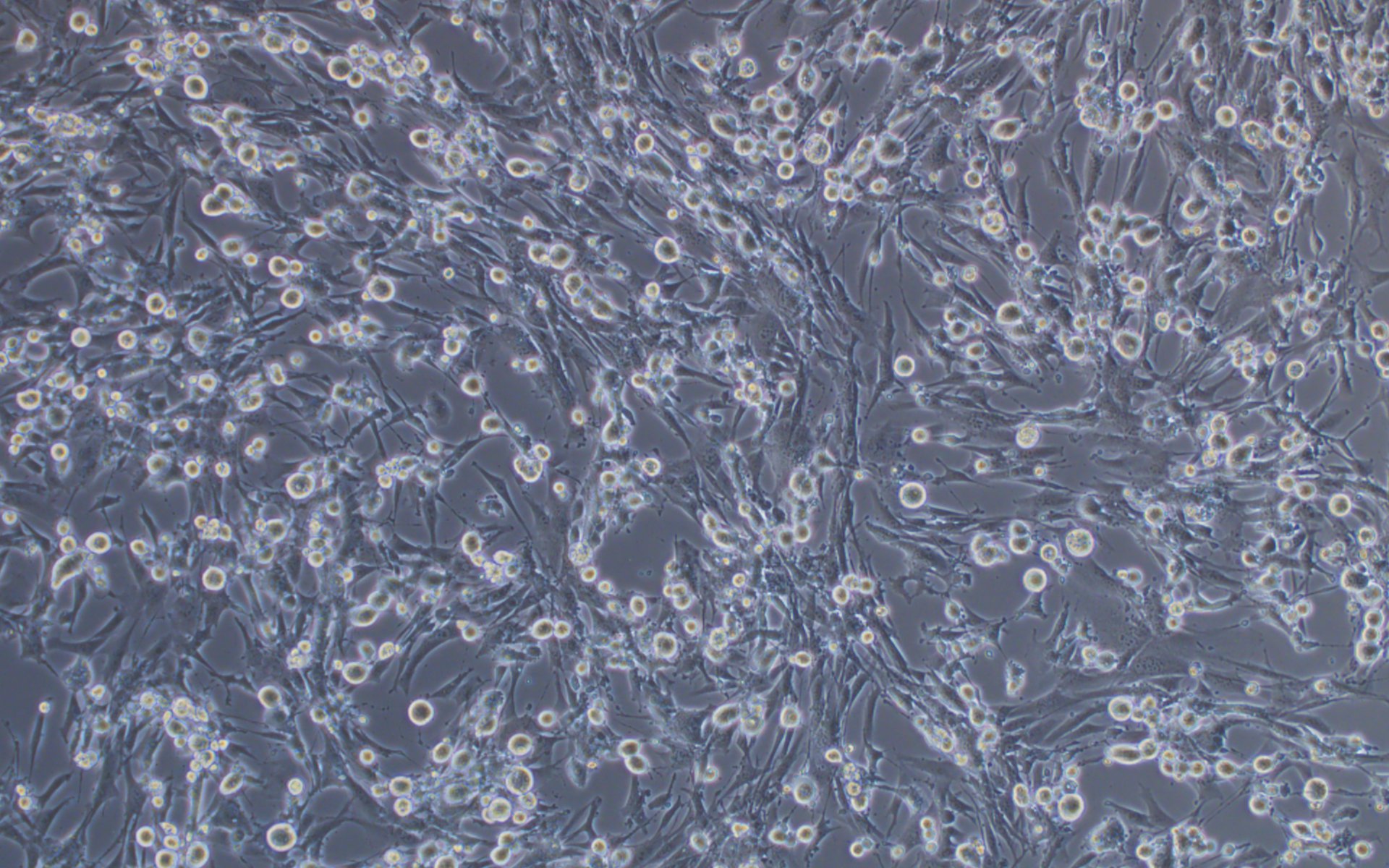
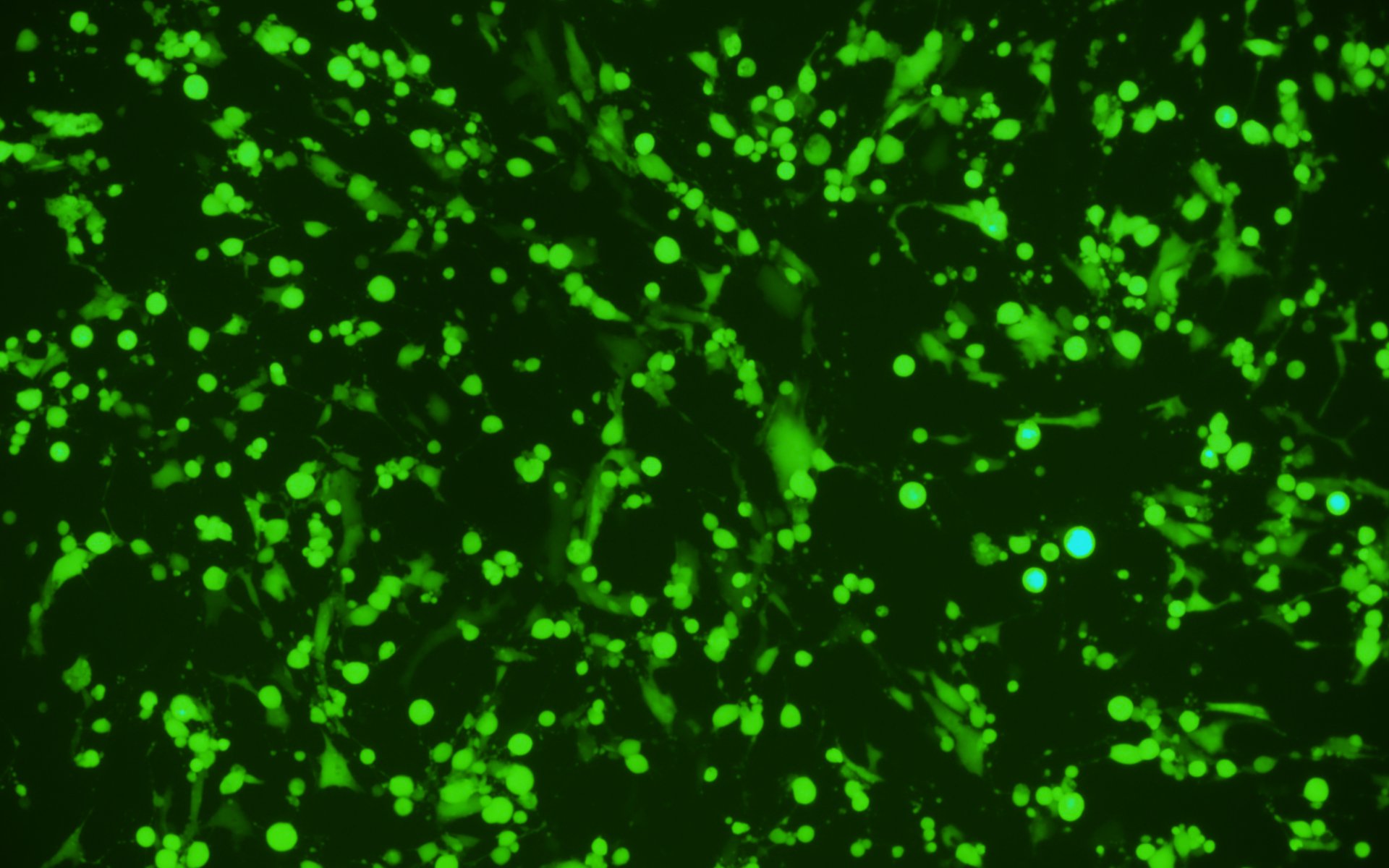
Figure 4. VSV pseudotyped with SARS-CoV-2 S protein and its D614G mutant specifically infected BHK-21 cells overexpressing human ACE2 receptor. Images were taken at 24 hours post-transduction. Magnification: 100x.
How to Order
Customer-supplied vectors
If customer-supplied VSV plasmids are used in packaging, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
Resources
FAQ
We use plaque assay for measuring VSV titer. Briefly, a monolayer of viral permissive cells is infected at a low dilution so that only a few cells get infected and overlayed with agarose to limit the spread of virus. Each infected cell lyses and infects the adjacent cells in turn, ultimately forming a group of infected cells known as a plaque. Since each plaque represents a single virus, the titer of a viral stock can be determined by counting the number of plaques obtained from varying dilutions of that stock.
Recombinant VSV vectors generated by VectorBuilder are deficient in replication due to deletion of the envelope glycoprotein G gene. The envelope proteins of heterologous viruses, including viruses requiring high-level containment can be supplied in trans for producing VSV pseudotypes. Since VSV pseudotypes are unable to undergo more than a single round of replication, they can be safely handled in a regular BSL2 facility. This makes VSV vectors highly suitable for studying cell entry mechanisms of high-risk viruses without the requirement of high-level containment.
The estimated turnaround is the time from production initiation to completion. It does not include waiting time for any customer-supplied materials (e.g. template DNA or viral vectors), QC of such materials, and transit time for shipping final deliverables to the customer.





