AAV Biodistribution Profiling
Recombinant adeno-associated virus (AAV) has recently emerged as a highly popular gene delivery vector for a wide range of gene therapy and vaccine applications. One essential consideration when evaluating the therapeutic potential and safety profile of an AAV vector is its distribution and persistence in various organs and tissues.
While some biodistribution studies have been reported for commonly used AAV serotypes, they are far from adequate. Existing studies are generally limited to organ-level resolution without probing different cell types at the sub-organ level. They also typically focus on intravenous injection without examining other routes of administration. Importantly, because most studies are done on rodents, the high degree of evolutionary and physiological divergence from humans casts serious doubt on whether the data can be extrapolated to humans. Additionally, many newly developed natural and synthetic serotypes lack biodistribution data altogether. Compounding these problems is the possibility that different vector sequences packaged into the same serotype can still have different biodistribution as different transgenes can provoke different cellular response including innate immunity.
The AAV biodistribution profiling services offered by VectorBuilder can overcome all these problems and help you obtain high-resolution data in the most appropriate species. Of particular note is our ability to conduct biodistribution profiling in non-human primates (NHPs). As described below, VectorBuilder can offer the most comprehensive AAV biodistribution studies in the industry.
Service Highlights
- Full-service platform: VectorBuilder is the world’s only company capable of providing one-stop solution for all your AAV preclinical and clinical CRO and CDMO needs. Our AAV services encompass vector design and optimization, vector cloning, virus packaging, capsid evolution and targeted engineering, AAV biodistribution profiling, and GMP manufacturing.
- Multiple species including nonhuman primate (NHP): We offer a wide range of species for our in vivo biodistribution studies, such as mouse, rat, and importantly, two nonhuman primate species: crab-eating macaque (Macaca fascicularis; a.k.a. cynomolgus monkey) and rhesus macaque (Macaca Mulatta). Studies are carried out in AAALAC-accredited facilities by highly trained professionals.
- Multiple analytical assays: We can perform a variety of assays to examine AAV biodistribution at the organ, tissue and cell level, including imaging of fluorescence reporters, isolation of fluorescence-positive cells by flow sorting, quantification of luciferase reporter activities, genome copy number analysis by qPCR, GOI expression analysis by RT-qPCR, etc.
- Multiplexing analysis using barcode and NGS: Many preclinical AAV pipelines need to compare the biodistribution of multiple types of vectors before selecting the most appropriate ones for further studies. This can arise from the same vector design packaged into different serotypes or different vector designs packaged into the same serotype. VectorBuilder developed a proprietary platform for assessing the biodistribution of different vectors within the same animal. Specifically, different barcodes are added to the vectors, and the distribution of either the genomic DNA or mRNA of the AAV vectors in a given tissue can be ascertained by next-generation sequencing (NGS) of the barcodes. This approach can significantly reduce cost and timeline, and more importantly, it allows the abundance of different vectors within a tissue to be used as internal references of each other, thus increasing the sensitivity and reliability of the data.
- In house availability of many AAV serotypes: We offer a variety of common serotypes for packaging your AAV vector (1, 2, 3, 4, 5, 6, 6.2, 7, 8, 9, rh10, DJ, DJ/8, PHP.eB, PHP.S, AAV2-retro, AAV2-QuadYF, AAV2.7m8, etc.). We can also quickly develop new serotypes per your requirements.
- Multiple routes of administration: Various AAV administration methods are available, including tail vein injection, facial vein injection (for neonatal mice and rats), intracerebroventricular injection, intrathecal injection, subretinal injection, intravitreal injection, intratympanic injection, intramuscular injection, and more.
- Full technical support: Our highly experienced scientists can provide comprehensive technical support covering every aspect of your AAV project, ranging from vector design and optimization to virus packaging including GMP manufacturing, from cell culture analysis to animal surgery.
Experimental Data
Below are a few examples illustrating our expertise in high-quality AAV production and AAV biodistribution profiling.
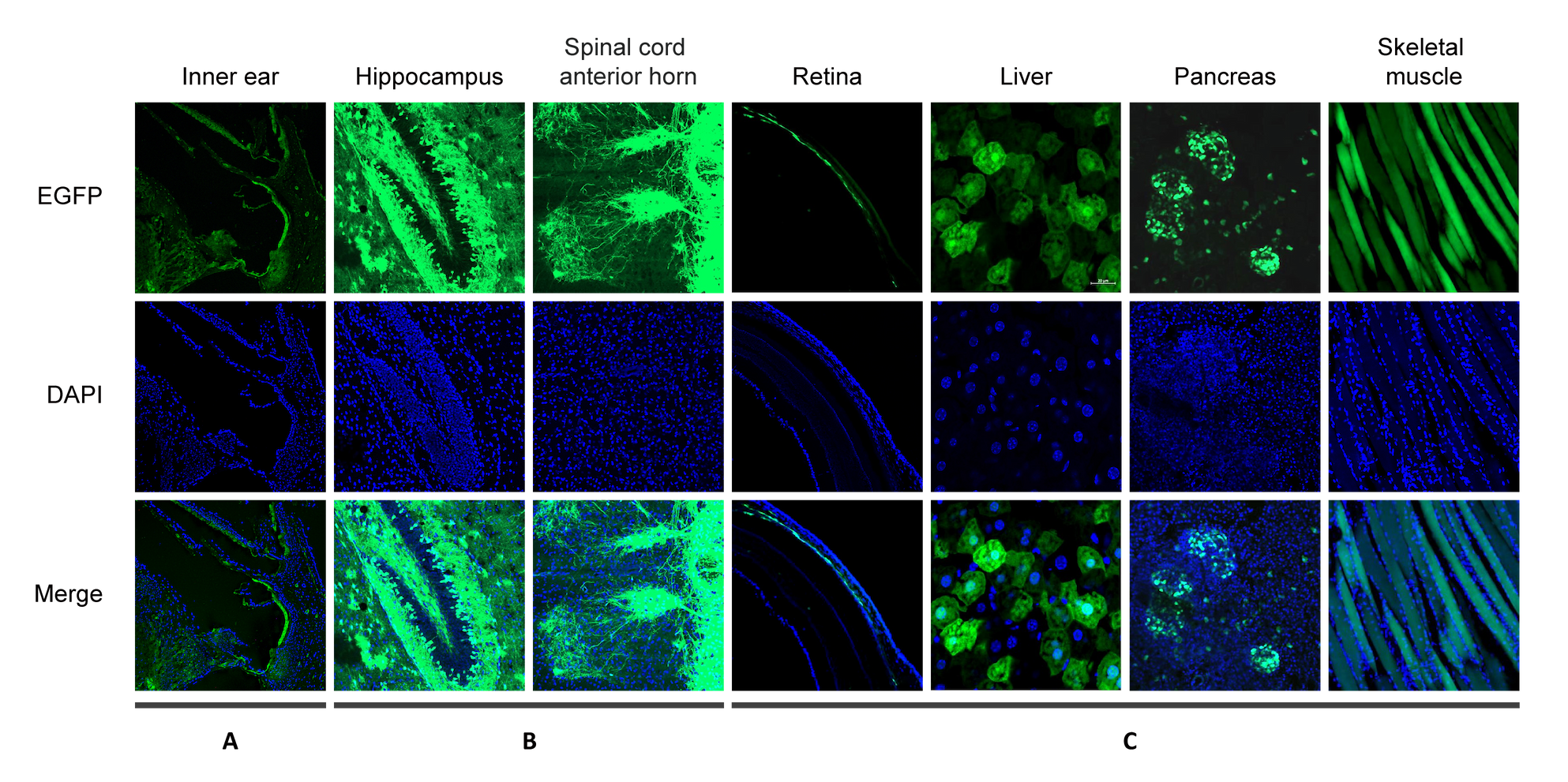
Figure 1. AAV9 carrying CAG promoter driving EGFP was administered to mice by various routes. EGFP and DAPI fluorescence was analyzed in the following organs: (A) inner ear, images were taken 13 days after vector delivery by intratympanic injection to the left ear; (B) hippocampus and spinal cord anterior horn, images were taken 10 days after vector delivery by facial vein injection; (C) retina, liver, pancreas, and skeletal muscle, images were taken 12 days after vector delivery by tail vain injection.
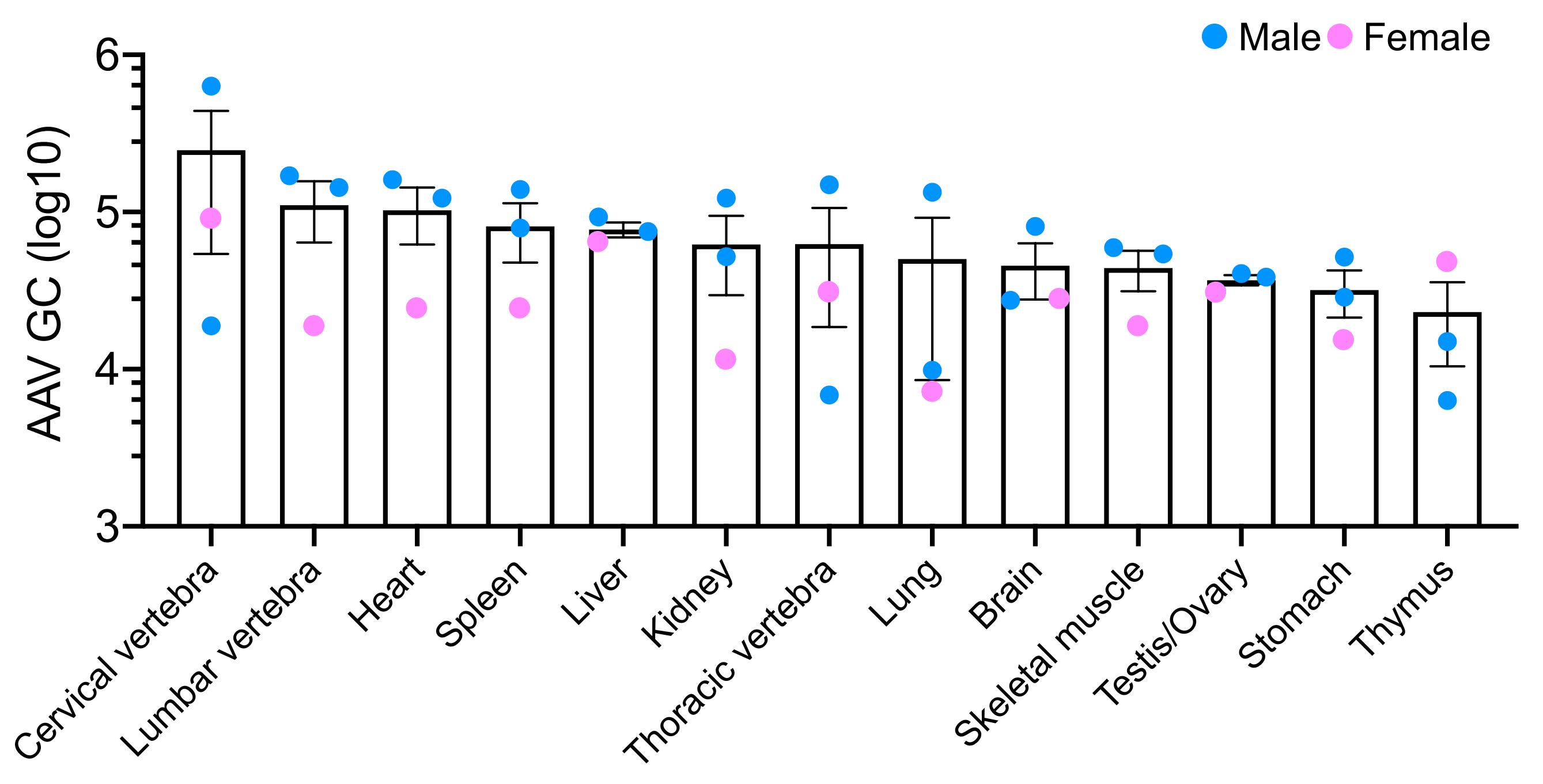
Figure 2. Recombinant AAV9 particles (~1.5X1010 GC/mouse) were intravenously administered to three neonatal mice (two male and one female) within 48 h after birth. AAV9 biodistribution in several organs was quantified using qPCR six weeks after the injection.
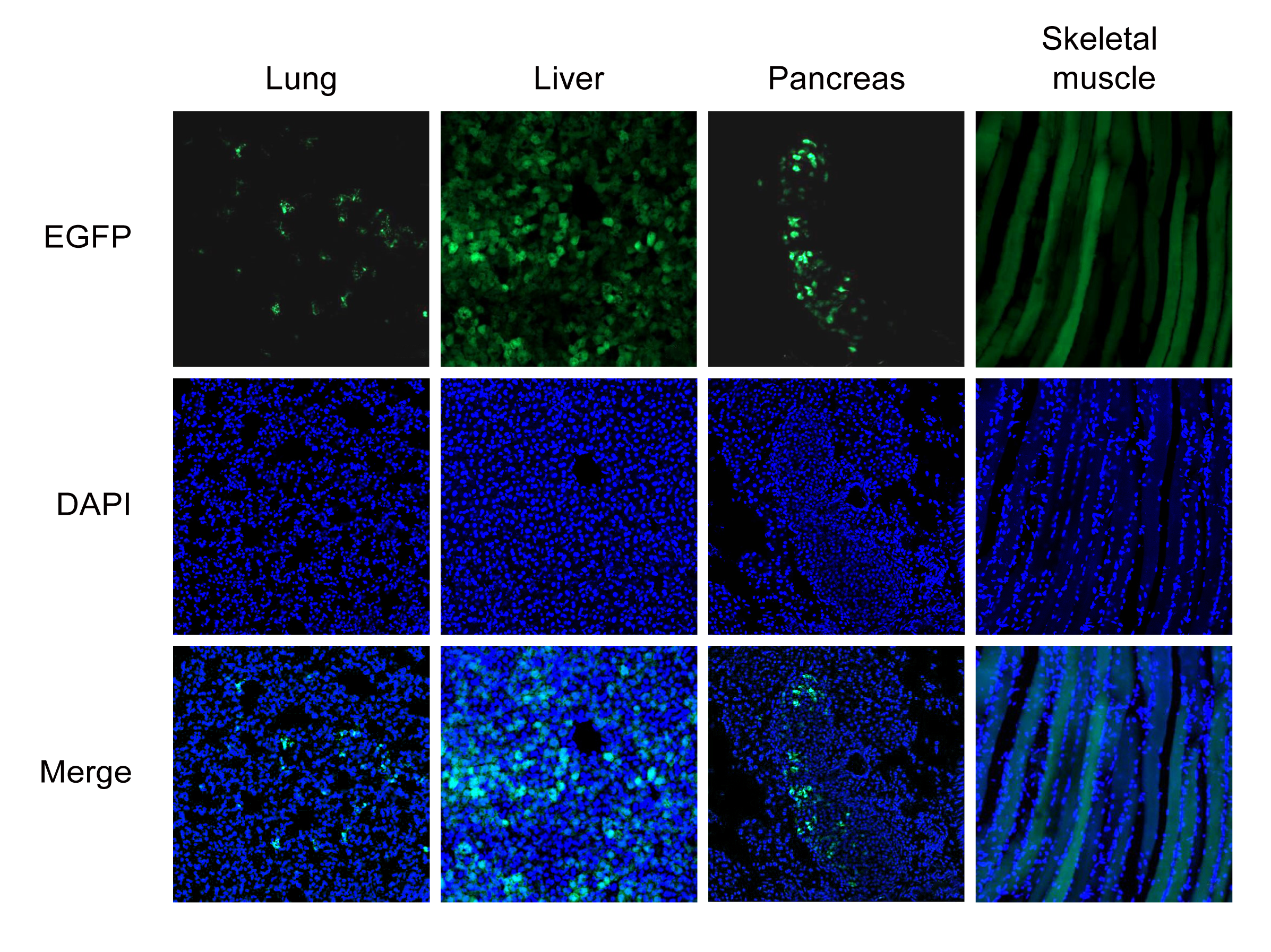
Figure 3. AAV1 carrying CMV promoter driving EGFP was administered to mice by tail vein injection. EGFP and DAPI fluorescence was analyzed in lung, liver, pancreas, and skeletal muscle. Images were taken 12 days after injection.
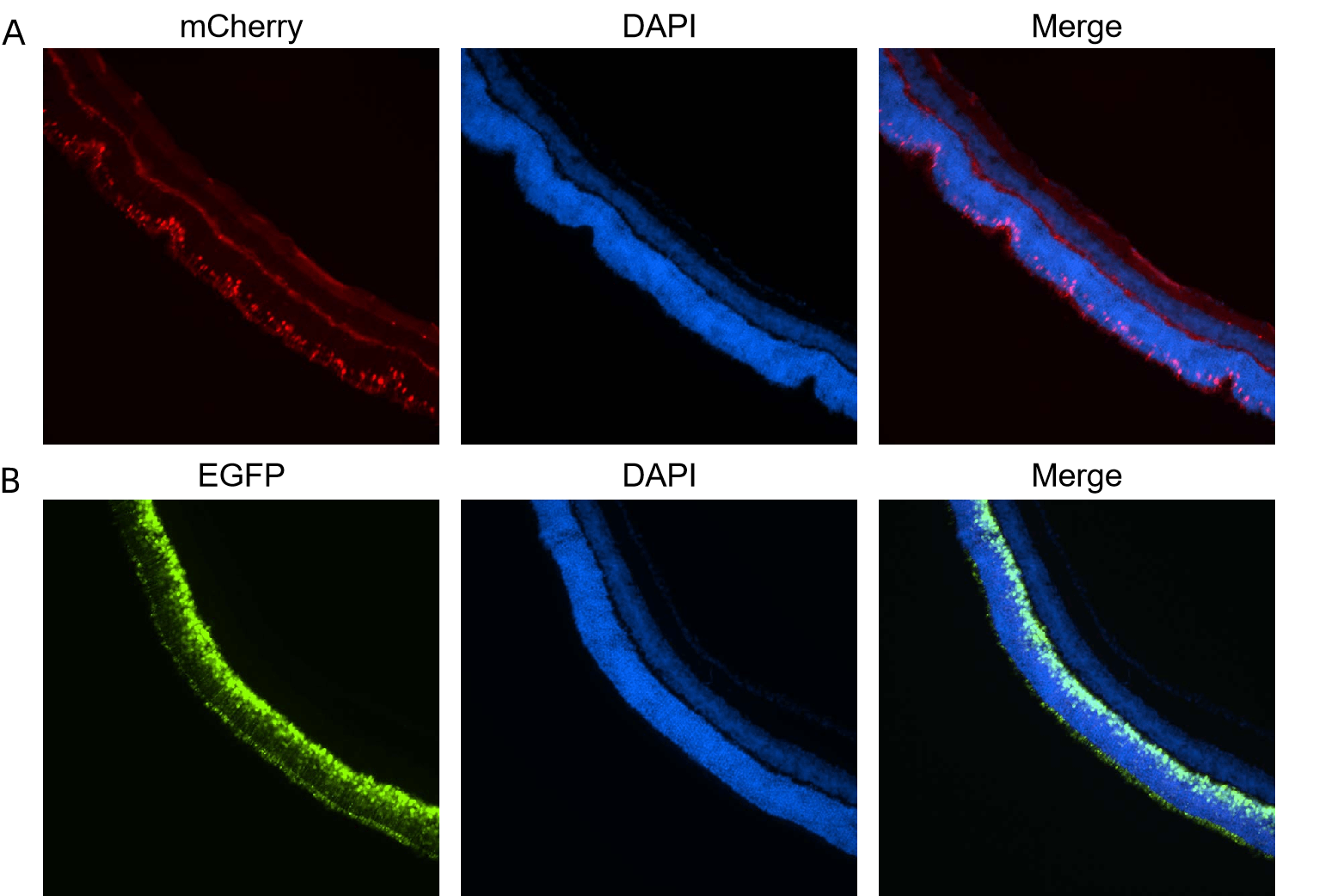
Figure 4. (A) AAV8 carrying ProA1 promoter driving mCherry or (B) AAV8 carrying hRHO promoter driving EGFP was administered to mice by subretinal injection. mCherry, EGFP, and DAPI fluorescence was analyzed in retina. Images were taken 20 days after injection.
To inquire about our CRO services email us at cro@vectorbuilder.com. For other questions contact us.




