Driving CAR-T Therapy in the Clinic and with CRISPR
Keywords: CAR-T cell therapy, Kymriah CAR-T, immunotherpy
CAR-T cell therapy is rapidly becoming a clinically viable option for cancer treatment, and innovations in the field are on the rise, with 6 FDA-approved CAR-T cell therapies currently available. This treatment utilizes reprogrammed immune cells which are derived from the patient's own body. Following editing and re-administration of these immune cells, they can specifically target and destroy invasive cancer cells, unlike conventional chemotherapies. In this post, we will delve into the basic biology behind CAR-T cell vectors and highlight a CAR-T cell therapy that is used in the clinic. We will also elaborate on how CRISPR/Cas9 is being used to create the next generation of CAR therapeutics.
CAR-T cell therapy
Therapy utilizing T cells expressing Chimeric Antigen Receptors (CAR), often abbreviated as CAR-T therapy, represents a revolutionary form of immunotherapy in the field of cancer treatment. This innovative approach involves genetically modifying a patient's own T-cells, a type of white blood cell crucial to the immune system, to express chimeric antigen receptors on their surface. These receptors are specifically designed to target and recognize unique markers, or antigens, present on cancer cells. Once these CAR-T cells are infused back into the patient's bloodstream, they become potent warriors, homing in on cancer cells with remarkable precision and launching a powerful attack against the disease. CAR-T therapy has demonstrated remarkable success in treating certain types of cancer, offering new hope to patients who previously had limited treatment options and showcasing the extraordinary potential of harnessing the immune system's natural capabilities to combat cancer.
CAR-T therapy hinges on the use of a synthetic receptor designed for immunotherapy applications. It consists of three main components: (1) an extracellular antigen recognition domain, usually derived from a single-chain variable fragment (scFv) of an antibody, which enables specific binding to a target antigen on cancer cells; (2) a transmembrane domain that anchors the CAR within the cell membrane; and (3) an intracellular signaling domain, often derived from the CD3ζ chain of the T-cell receptor complex, along with one or more co-stimulatory domains like CD28 or 4-1BB. This unique structure allows CAR-T cells to recognize and target cancer cells independently of the major histocompatibility complex (MHC), leading to the activation of cytotoxic activity of the T-cells against the tumor.
Recognizing the target cell independently of the MHC is a fundamental advantage in the context of CAR therapy. Firstly, this feature allows this therapy to be applicable across a broad spectrum of cancer types. Traditional immune responses depend on the presentation of antigen fragments by MHC molecules, and some cancer cells can evade this process by downregulating or altering MHC expression. CAR-T cells, on the other hand, are engineered to recognize surface antigens directly, bypassing the need for MHC presentation. Consequently, CAR-T cells can effectively target cancer cells that may otherwise remain hidden from the immune system's natural surveillance. This adaptability is critical in combating the heterogeneity of tumors, as different cells within a tumor may exhibit varying MHC expression levels.
Secondly, CAR-T cell therapy offers enhanced specificity and reduced off-target effects. By precisely recognizing specific surface antigens on cancer cells, CAR-T cells minimize the risk of attacking healthy cells. This precision is especially important in preventing severe side effects commonly associated with traditional immunotherapies that rely on MHC-restricted T-cell recognition. In summary, the ability to target cancer cells independently of MHC status ensures CAR-T cell therapy's effectiveness, enabling it to address a wide range of cancers, overcome immune evasion strategies, maintain specificity, and provide a versatile and standardized treatment approach.
Kymriah (tisagenlecleucel)
Kymriah, known by its generic name Tisagenlecleucel, is a CAR-T cell therapy developed by Novartis that first received FDA approval in 2017. It was specifically designed to treat pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and its use has been expanded to Adult Diffuse Large B-cell Lymphoma (DLBCL) and Adult Follicular Lymphoma (FL). The treatment process for Kymriah begins with apheresis of the patient's blood, which removes plasma containing T-cells, followed by re-administration of the blood. After apheresis, the cells are cryopreserved and shipped to a manufacturing facility.
The patient's cells undergo incubation with immune-activating magnetic beads, which is followed by the delivery of a lentiviral vector. This vector serves as a mechanism for the permanent integration of a genetically engineered cassette into the cellular DNA. This cassette encodes the CAR molecule, a composite structure with discrete components. The CAR consists of an extracellular domain designed to recognize CD19, a specific antigen prevalent on the surface of cancerous B cells. The CAR also possesses a transmembrane domain, anchoring it within the T cell's membrane, ensuring structural stability. Furthermore, an intracellular signaling domain within the CAR plays a critical role in initiating a series of events upon antigen binding, leading to T-cell activation and the subsequent destruction of the targeted cancer cell. This intricate process underpins Kymriah's approach to cancer therapy, representing a promising avenue for leukemia and lymphoma treatment by harnessing the body's immune system.

Figure 1. Cartoon representation of Kymriah chimeric antigen receptor
The clinical trials for Kymriah yielded highly promising outcomes, particularly in patients with relapsed/refractory pediatric and young adult ALL. In these trials, Kymriah demonstrated an impressive overall response rate (ORR) of 82%, including complete remissions. Furthermore, the 5-year relapse-free survival reached a remarkable 49%, signifying prolonged disease-free periods and durable responses. Similarly, in patients with relapsed/refractory DLBCL and FL, Kymriah exhibited substantial efficacy, with ORRs of 53% and 86%, respectively, offering renewed hope to individuals facing challenging treatment scenarios in these hematological malignancies.
Improving CARs with CRISPR
Despite the advancements with CAR-T therapy, the field still faces challenges in manufacturing and in vivo persistence of the cells. Limited expansion and persistence of CAR T-cells can lead to treatment failure. Additionally, issues with collecting autologous T-cells and the hostile tumor microenvironment hinder therapy effectiveness, especially in solid tumors. Genetic editing techniques using CRISPR/Cas9 offer potential solutions to these barriers by enhancing CAR T-cell therapy.
CRISPR/Cas9 technology allows precise genome editing by creating double-stranded DNA breaks at specific sites. This system has been harnessed for various applications in gene editing and these tools can be applied to enhance CAR T-cell therapy by addressing various challenges, such as improving T-cell function, reducing toxicity, and increasing availability.
One major hurdle is the phenomenon of T-cell exhaustion, where prolonged exposure to tumor antigens leads to a loss of T-cell functionality. CRISPR/Cas9 provides a means to combat this exhaustion by precisely targeting inhibitory receptors such as PD-1 and CTLA-4, potentially rejuvenating CAR-T cells and enhancing their therapeutic efficacy.
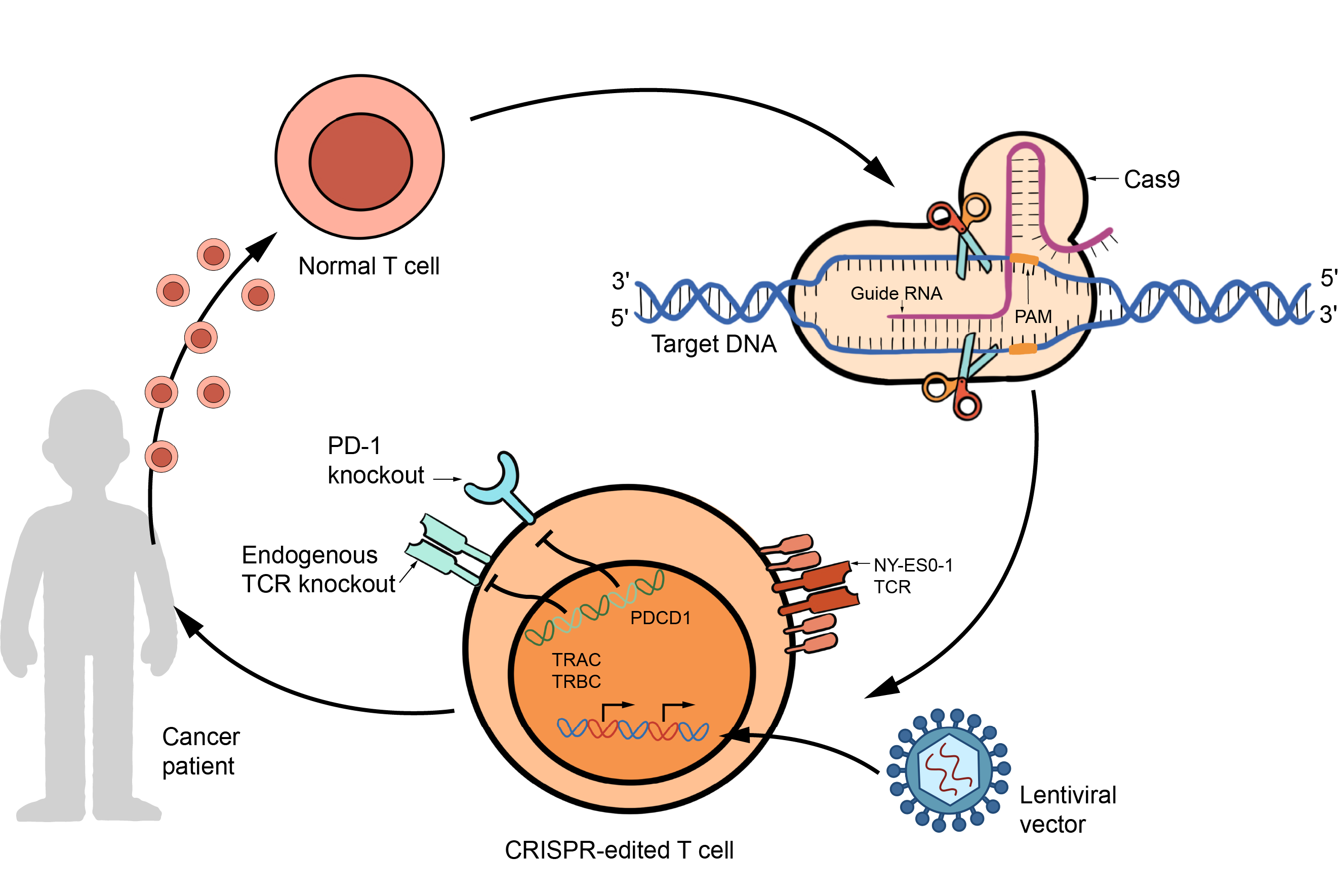
Figure 2. Overview of CRISPR/Cas9 technology use in CAR-T cells.
Moreover, CRISPR-Cas9 offers avenues to optimize the function and safety of CAR-T cells. By modulating cytokine signaling, gene editing can fine-tune the activation and expansion of CAR-T cells, bolstering their antitumor activity while mitigating toxic side effects. This technology also enables the precise integration of CAR transgenes into specific genomic locations, ensuring consistent and controlled CAR expression. Such targeted integration reduces the risk of tonic signaling, a phenomenon that can impair CAR-T cell function and durability.
Furthermore, CRISPR-Cas9 revolutionizes the production of CAR-T cell therapies by enabling the creation of "off-the-shelf" universal CAR-T cell products. Allogeneic T-cell therapies, derived from healthy donor cells, can be modified to resist rejection by host immune cells through gene editing. This approach simplifies the manufacturing process, making CAR-T cell therapy more accessible, cost-effective, and efficient, particularly for patients lacking an adequate supply of autologous T-cells.
In conclusion, CAR-T cell therapy has emerged as a powerful tool in the fight against cancer, demonstrating remarkable efficacy in treating various malignancies. The success of therapies like Kymriah underscores the potential of harnessing the patient's immune system to target cancer cells with precision. However, the integration of CRISPR/Cas9 gene editing technology holds the key to further enhancing CAR-T therapy's effectiveness. By addressing challenges such as T-cell exhaustion, manufacturing efficiency, and in vivo persistence, CRISPR/Cas9 offers a pathway to unlock even greater therapeutic potential. Through precise genome editing, we can envision a future where CAR-T cell therapy becomes more accessible, adaptable, and efficacious, bringing us closer to conquering cancer and providing new hope to patients worldwide.
Source
Dimitri, A., Herbst, F. & Fraietta, J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer 21, 78 (2022). https://doi.org/10.1186/s12943-022-01559-z
Awasthi R, Maier HJ, Zhang J, Lim S. Kymriah® (tisagenlecleucel) - An overview of the clinical development journey of the first approved CAR-T therapy. Hum Vaccin Immunother. 2023 Dec 31;19(1):2210046. doi: 10.1080/21645515.2023.2210046. Epub 2023 May 15. PMID: 37185251; PMCID: PMC10294746.



