Unleashing the Power of Oncolytic Viruses: Design Strategies for Maximum Efficacy
Keywords: Oncolytic virus, tumor targeting, immune stimulation
Oncolytic viruses offer a promising approach to cancer therapy, harnessing the intrinsic properties of viruses to specifically infect and lyse tumor cells and activate the immune system. Unlike traditional chemotherapy, which can be cytotoxic to normal and cancerous cells, a more targeted approach is achieved through genetically engineering viruses to thrive within the tumor microenvironment (TME) while sparing normal cells. A number of viruses have been utilized in oncolytic virus research, including vaccinia virus (VACV), herpes simplex virus (HSV), adenovirus and vesicular stomatitis virus (VSV). In this article, we will focus on the key attributes of two of the most promising oncolytic viruses, namely VACV and HSV, and delve into the different design strategies used to enhance therapeutic efficacy.
The key to making a potent oncolytic virus is: Choosing a virus with strong lytic activity whose genome can be edited to select for tumor cells combined with optimal recombinant virus design, including genetic modification to enhance virus survival, tumor-specific targeting, and stimulation of a strong anti-tumor response.
What makes a virus effective as an oncolytic agent?
Important considerations must be made when assessing the therapeutic potential of an oncolytic viral system, including tropism, lytic potential, and the safety profile of the virus. Despite a plethora of research on many different oncolytic viruses, only a handful of oncolytic viruses have been approved for clinical use, of which two are HSV-1. HSV has several key attributes that have made it the most commonly used oncolytic virus for cancer therapy. HSV can infect many different cell types, including neurons, and exhibits a natural tropism for some cancer cells due to the overexpression of HSV receptors (nectin-1 and herpes entry mediator) on tumor cells. Following infection, HSV can rapidly spread throughout tumor cells due to its short life cycle which promotes cell lysis and further rounds of infection. Although wild-type HSV is pathogenic to humans, the virus can be attenuated through modification of its genome to inhibit efficient viral replication in normal cells, and effective antiviral treatments can further enhance this safety.
While HSV-1 has demonstrated success in the clinic, VACV poses some clear advantages over HSV as an oncolytic agent, despite there being no current clinically approved treatments (Table 1). Similar to HSV, VACV also demonstrates a natural tropism for cancer cells due to the overexpression of a protein which promotes viral replication, specifically the epidermal growth factor receptor (EGFR). However, VACV displays a broader tropism than HSV-1, facilitated by direct cellular entry through the fusion of the viral lipid membrane with the host cell membrane, bypassing the need for a receptor altogether. Furthermore, VACV, like HSV, can rapidly spread throughout tumors inducing cell lysis. VACV exhibits a good safety profile confirmed by extensive research and data for its clinical use as the smallpox vaccine. Modern VACV strains have undergone directed evolution to enhance lytic potential (e.g. the Western Reserve strain exhibits high oncolytic activity), and like HSV, its genome can be modified to attenuate the virus to limit replication in normal cells.
One of the major advantages of VACV over HSV-1 is reduced susceptibility to clearance by the host antibody response. This is particularly advantageous in the clinic, whereby VACV can be administered intravenously and maintain high efficacy, compared to HSV which is likely to be cleared by the host immune response. Instead, HSV-based drugs, such as TVEC, are administered via direct injection into the tumor, which is a complex and invasive procedure with increased infection risks. This also limits current HSV-based drugs to specific cancer types that are easily accessible (e.g. sarcoma and melanoma), whereas VACV is better suited for targeting difficult to reach sites (e.g. pancreatic and liver cancers) and metastasized cancers. Despite the challenges associated with intravenous injection of oncolytic HSV, a recent Phase 1 clinical trial demonstrated great promise for the future of intravenous administration of HSV-based drugs.
| VACV | HSV | |
|---|---|---|
| Approved medical oncolytic virus | × | TVEC, DELYTACT |
| Broad tropism | ✓ | ✓ |
| Resistant to clearance by host immune response | ✓ | × |
| Main routes of administration | Intratumoral + Intravenous | Intratumoral |
| Overall safety | ✓ | ✓ |
Table 1. VACV vs HSV for oncolytic virotherapy. Boxes are highlighted in green to demonstrate which virus performs better for each category.
Designing a potent oncolytic virus
When designing an oncolytic virus, choosing VACV or HSV for recombinant virus engineering is a good place to start. Both recombinant viruses are highly amenable to genetic modifications that can greatly enhance their efficacy and/or safety as oncolytic agents. One particular benefit of VACV and HSV is their large genome size of 190 kb and 150 kb, respectively, enabling them to accommodate large genetic inserts. A major difficulty that arises when working with genomes of this length is cloning and genetic engineering. Conventional plasmids cannot be utilized; instead, other cloning systems are used, such as the bacterial artificial chromosome (BAC) and the yeast artificial chromosome (YAC). A combination of the key elements from both can be used (BACYAC) to enable growth and modification of the vector in either E. coli or yeast. The strategic design of this large space can significantly improve specificity for targeting cancer cells, enhance tumor-destroying capabilities, and bolster safety profile, overall greatly increasing effectiveness as cancer therapies.
Enhanced targeting
Researchers have commonly employed cell-targeting inserts or specific mutations that enhance tumor cell specificity and reduce off-target side effects in oncolytic viruses, making them more efficacious in clinical applications (Figure 1). These changes are alterations of the viral genome, which when combined with the expression of a therapeutic transgene, allow for cancer cell specific infection and lysis.
Enhanced tumor specificity can be achieved by incorporating inserts or mutations that enhance viral binding to cancer receptors or reduce binding to normal cells. For oncolytic HSV, expressing an anti-HER2 antibody in place of the endogenous receptor binding domain of glycoprotein D, facilitates binding of the virus to cancer cells which overexpress HER2 (e.g. in breast, ovarian and glioblastoma cancers).
Deleting genes from the viral genome encoding proteins that are specifically over-expressed in tumor cells and essential for efficient viral replication, can enhance specificity by ensuring the virus can only efficiently replicate in cancer cells. For example, deletion of a viral gene encoding an enzyme essential for DNA metabolism, namely thymidine kinase (TK), has proven successful for the VACV-based drug JX-594. Host TK can compensate for the loss of viral TK, but only in cancer cells where this protein is overexpressed. When designing HSV-based drugs, it is wise to retain the TK gene, ensuring the virus is susceptible to the antiviral nucleoside analog drugs ganciclovir and acyclovir. Instead, replication in normal cells can be attenuated by mutating the viral gene encoding ICP6, an enzyme essential for viral genome synthesis. Compensation for the loss of viral ICP6 by host cells has been shown to only occur in dividing cells, such as cancer cells.
Another approach to enhance targeting of oncolytic virus replication is to hamper evasion of the host immune response, ensuring only efficient replication within the TME, where host immunity and translational shutdown are suppressed. A common mutation utilized for oncolytic HSV-based drugs is mutation of the γ34.5 gene, whose encoded protein inhibits translation initiation and plays an important role in viral resistance to the host IFN response. Consequently, the γ34.5 mutation disrupts both viral immune evasion and viral resistance to host translational shutdown, hampering viral replication in normal cells outside of the TME.
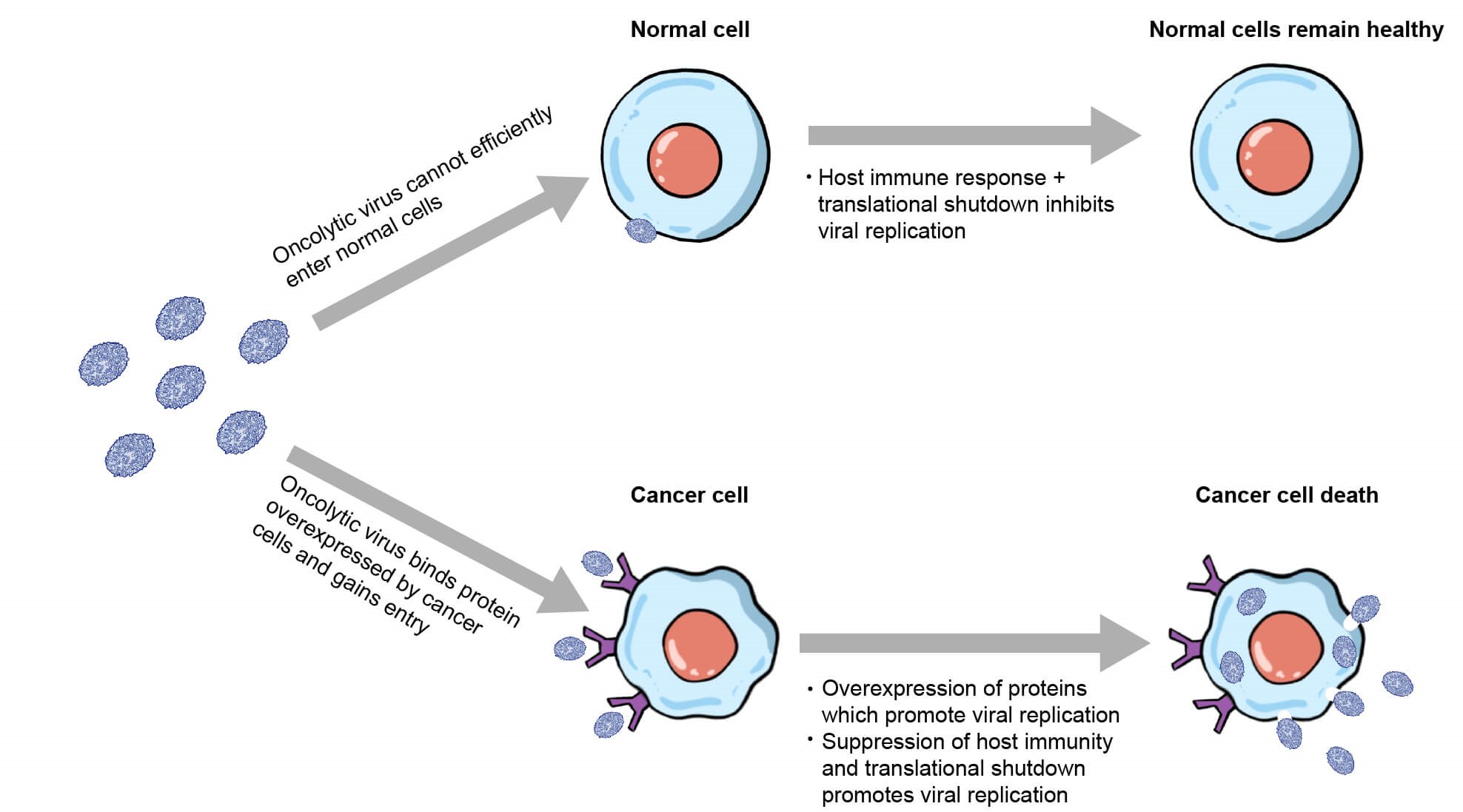
Figure 1. Diagram of oncolytic virus displaying enhanced tropism for cancer cells.
Enhancing tumor destruction: anti-angiogenic effects
Another important consideration when designing an oncolytic virus is how to enhance the efficacy of virus-induced tumor destruction. Cancer cell death can result as a direct consequence of oncolytic virus replication inducing cell lysis. However, many other indirect effects of viral infection play an important role in facilitating tumor destruction, which can be exploited to enhance treatment success. This can be accomplished by encoding a transgene in the BACYAC backbone which targets angiogenic and/or immune activities.
Angiogenesis is important for tumor survival and growth, and inhibiting this process essentially starves the tumor of essential nutrients. The VACV-based drug JX-594 infects tumor-associated vascular endothelial cells, leading to vascular collapse due to direct damage to endothelial cells, clot formation, and the recruitment of neutrophils (Figure 2). When designing an oncolytic virus, the incorporation of genes which further enhance anti-angiogenic effects should be considered as a strategy to enhance tumor killing. One approach is to target pro-angiogenic host factors, such as the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). This can be achieved through the expression of genes encoding antibodies against EGFR and VEGF in the recombinant viral genome. This strategy has proven successful for oncolytic VACV, improving therapeutic efficacy in mice by disrupting ligand-receptor binding.
Although enhancing the anti-angiogenic effects of oncolytic viruses has proven a successful strategy, there is a fine balance between tumor destruction and promoting viral spread. By disrupting the blood supply, viral spread to other tumor sites may be disrupted, and the administration of other antitumor drugs, like chemotherapy, may also be affected. This is also an important factor which must be considered in the initial stages of oncolytic virus design to ensure efficacy as a therapeutic drug.
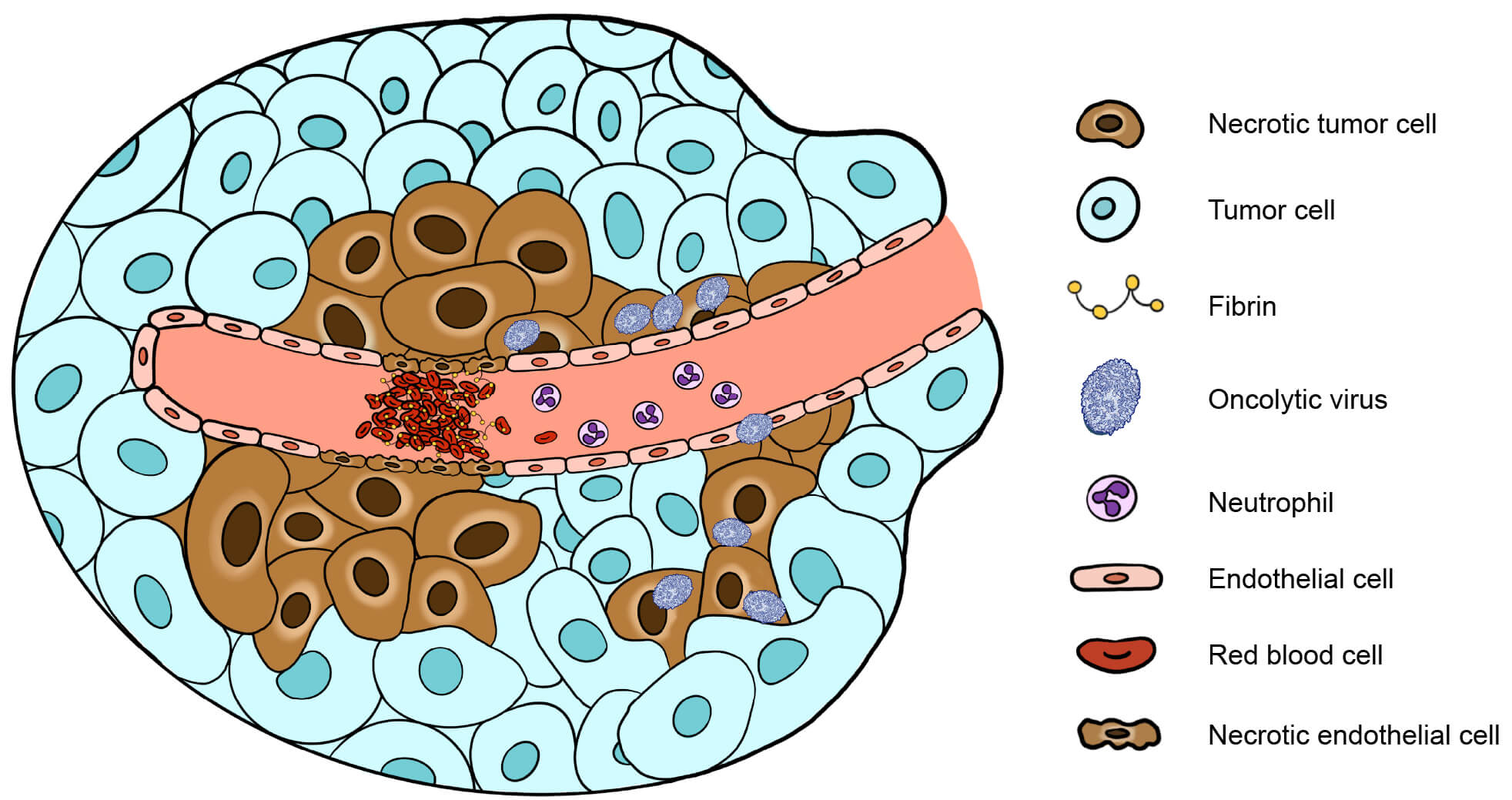
Figure 2. Mechanism of oncolytic virus-induced anti-angiogenic effects on tumors.
Enhancing tumor destruction: anti-tumor immunity
When designing an oncolytic virus to enhance tumor destruction, the overexpression of proteins, which enhance the ability of oncolytic viruses to overcome the immunosuppressive nature of the TME and mount an effective anti-tumor immune response, serves as a valuable approach. This is important for long-term anti-tumor effects and can be highly advantageous in a clinical setting, increasing the number of immune cells, pro-inflammatory cytokines, and chemokines at the tumor site, and thus priming tumors for successful immunotherapy treatment (e.g. with immune checkpoint inhibitors or adoptive cell transfer therapies such as CAR-T).
One approach is to insert genes encoding pro-inflammatory cytokines into the viral genome to recruit immune cells to the normally immune exhausted TME. HSV-based TVEC and VACV-based JX-594 are modified to encode the pro-inflammatory cytokine GM-CSF, which enhances the efficacy of antigen-presenting cells and, consequently, recruitment of T cells and NK cells to the tumor site. Other pro-inflammatory cytokines, such as IL-12 encoded by oncolytic HSV, have proven effective in enhancing the cytotoxic T-cell response and in stimulating IFN-γ production. Oncolytic HSV expressing IL-12 (M032) has shown promise for treating recurrent glioblastoma and has entered phase 1 clinical trials.
Another approach is to modify oncolytic viruses to overexpress proteins on cancer cells which are recognized by T cells. CAR-T cell therapy relies on the modification of patients’ T cells to express a CAR which recognizes its ligand (CD19) overexpressed on the surface of tumor cells. To enhance CAR-T therapy and prime the patient’s immune system to mount a more efficacious anti-tumor immune response, VACV can be used to overexpress a non-signalling, truncated version of CD19 on the surface of cancer cells. Similarly, VACV can be modified to express a secretory bispecific T-cell engager (BiTE), consisting of an antibody that recognizes a T-cell surface antigen (e.g. CD3) linked to an antibody that targets a tumor cell surface antigen (e.g. EphA2). In both cases, T cells are directed to tumors, leading to an overall enhancement in anti-tumor immunity (Figure 3).
It is important to consider the potential pitfalls of this design strategy, where a fine balance exists between stimulating an effective anti-tumor immune response without hampering viral spread. This is particularly important in the context of oncolytic virus therapy, especially when utilizing viruses such as VACV which can target metastatic cancer throughout the body.
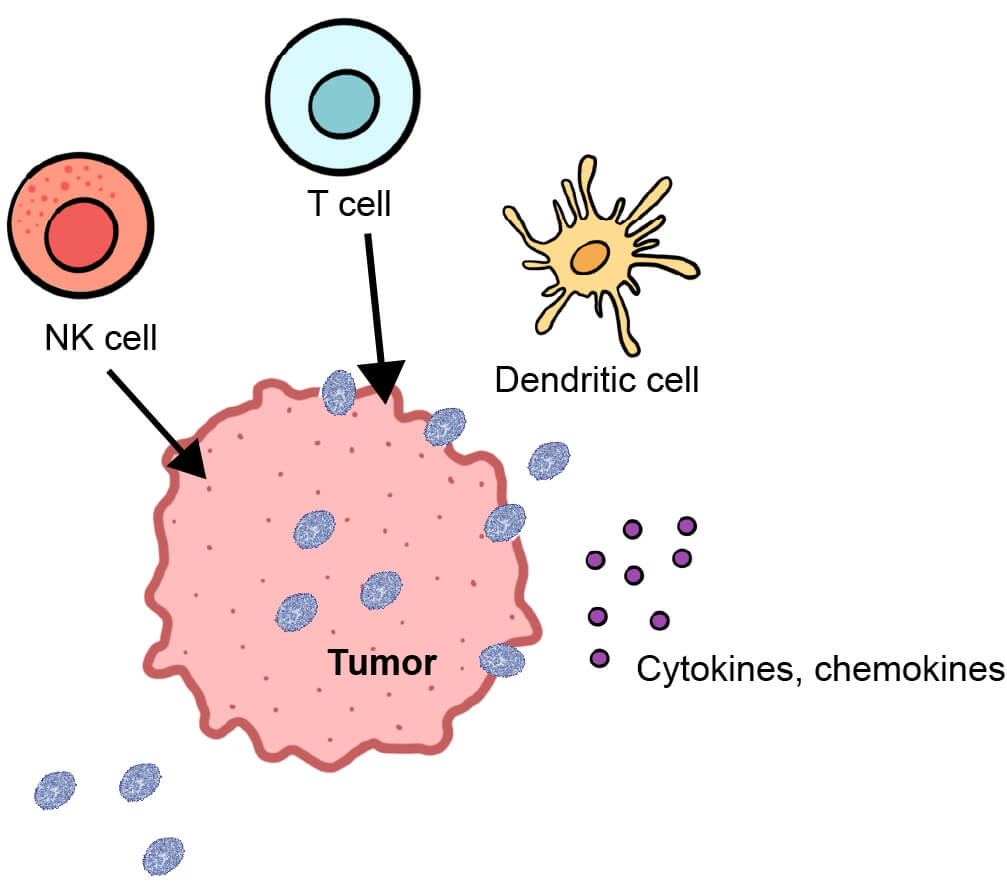
Figure 3. Diagram of anti-tumor immunity triggered by oncolytic virus infection.
Conclusion
Overall, VACV and HSV are highly effective oncolytic agents in the fight against cancer. Utilizing these recombinant viruses and taking advantage of their large cargo capacity allows us to not only rely on the natural oncolytic ability of these viruses, but also to enhance their efficacy through modification of targeting, angiogenesis, and the immune response. Creating these well-designed oncolytic viruses can present many challenges, including difficulty associated with cloning large inserts into the BACYAC backbone. However, VectorBuilder has extensive experience in challenging cloning projects and can ensure that your virus is produced with the highest accuracy and efficiency. An ever-increasing arsenal of knowledge, combined with access to more advanced genetic tools, offers exciting opportunities for the future development of more targeted and efficacious oncolytic virus therapies.
References
Lin, D., Shen, Y. & Liang, T. Oncolytic virotherapy: basic principles, recent advances and future directions. Sig Transduct Target Ther 8, 156 (2023).
Zhu X, Fan C, Xiong Z, Chen M, Li Z, Tao T, Liu X. Development and application of oncolytic viruses as the nemesis of tumor cells. Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. PMID: 37440883; PMCID: PMC10335770.
Tang G, Wang D, Zhao X, Feng Z, Chen Q, Shen Y. The Dilemma of HSV-1 Oncolytic Virus Delivery: The Method Choice and Hurdles. Int J Mol Sci. 2023 Feb 12;24(4):3681. doi: 10.3390/ijms24043681. PMID: 36835091; PMCID: PMC9962028.
Kaufman, H., Kohlhapp, F. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14, 642–662 (2015). https://doi.org/10.1038/nrd4663
Peters C, Rabkin SD. Designing Herpes Viruses as Oncolytics. Mol Ther Oncolytics. 2015;2:15010–. doi: 10.1038/mto.2015.10. Epub 2015 Jul 22. PMID: 26462293; PMCID: PMC4599707.
Xu L, Sun H, Lemoine NR, Xuan Y, Wang P. Oncolytic vaccinia virus and cancer immunotherapy. Front Immunol. 2024 Jan 12;14:1324744. doi: 10.3389/fimmu.2023.1324744. PMID: 38283361; PMCID: PMC10811104.
Liu X, Zhao J, Li X, Lao F, Fang M. Design Strategies and Precautions for Using Vaccinia Virus in Tumor Virotherapy. Vaccines (Basel). 2022 Sep 17;10(9):1552. doi: 10.3390/vaccines10091552. PMID: 36146629; PMCID: PMC9504998.



