The Basics of mRNA Therapeutics
Keywords: mRNA structure, lipid nanoparticles, mRNA vaccine
mRNA therapeutics are emerging in popularity, thanks in part to their widespread use in COVID-19 vaccination, but what exactly makes mRNA a good therapeutic? To answer that question let’s first dive into the basics of an mRNA molecule.
mRNA structure and features
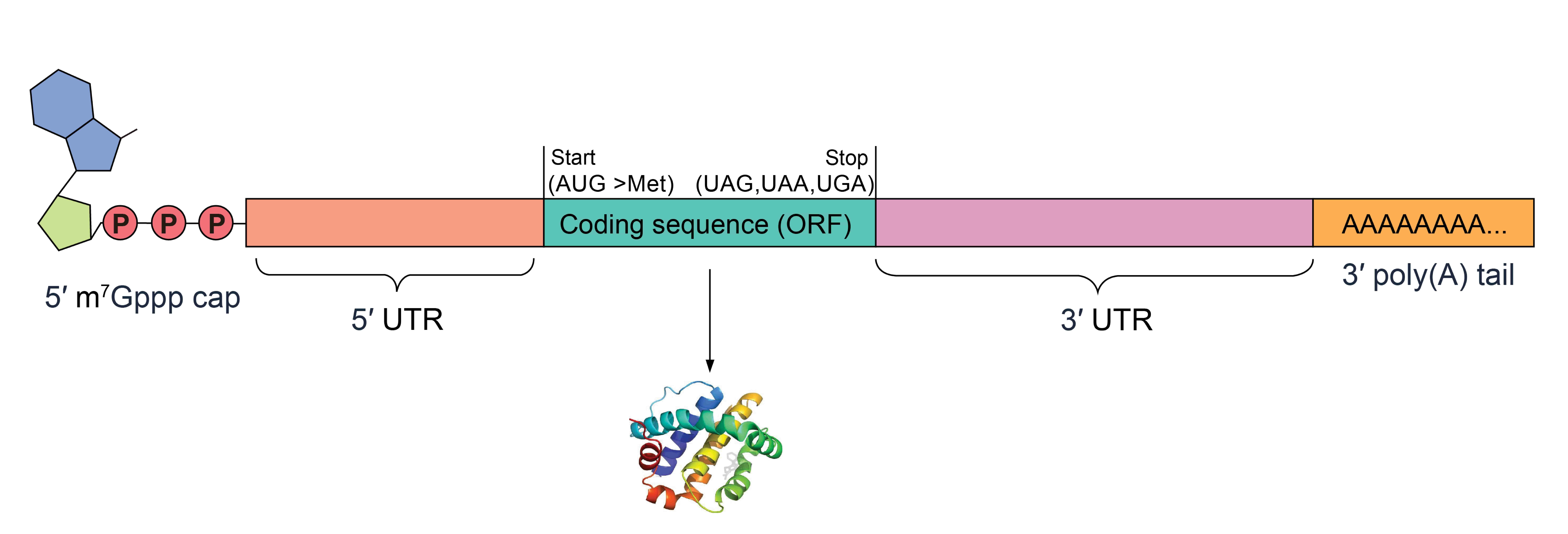
Figure 1. Diagram of an mRNA molecule
Moving from left to right in
The next critical portion of an mRNA molecule is the 5’ and 3’ untranslated region (UTR). These nucleotides are coded in a gene; however, they are not translated by the ribosome into functional protein. These portions of the mRNA act as targets for regulatory elements such as microRNAs and they can form secondary structures to aid in translation efficiency.
The actual coding sequence of an mRNA molecule falls in between the 5’ and 3’ UTRs of the molecule and begins with a start codon (AUG) and ends with translation termination at a stop codon (e.g. UGA). mRNA molecules consist of the nucleobases Adenine, Guanine, Cytosine, and Uracil. One fundamental aspect of an effective mRNA vaccine is the ability to elicit an antigen specific immune response, while mitigating non-antigen specific immune side reactions. Due to the innate immune system, our cells can recognize foreign mRNA, which results in a variety of unwanted immune side effects.
One of the ways to mitigate unwanted side effects of foreign RNA is to incorporate modified nucleotides into the reaction. Shown in Figure 2, two of the most common modified nucleotides used in RNA vaccination are 5-methylcytosine and N1-methylpsuedouracil. These nucleotides maintain proper Watson-Crick base pairing while altering the ability of the cell’s innate immunity to recognize the molecules as foreign. Both nucleotides were initially discovered in tRNAs but have since been exploited for incorporation into mRNA therapeutics.
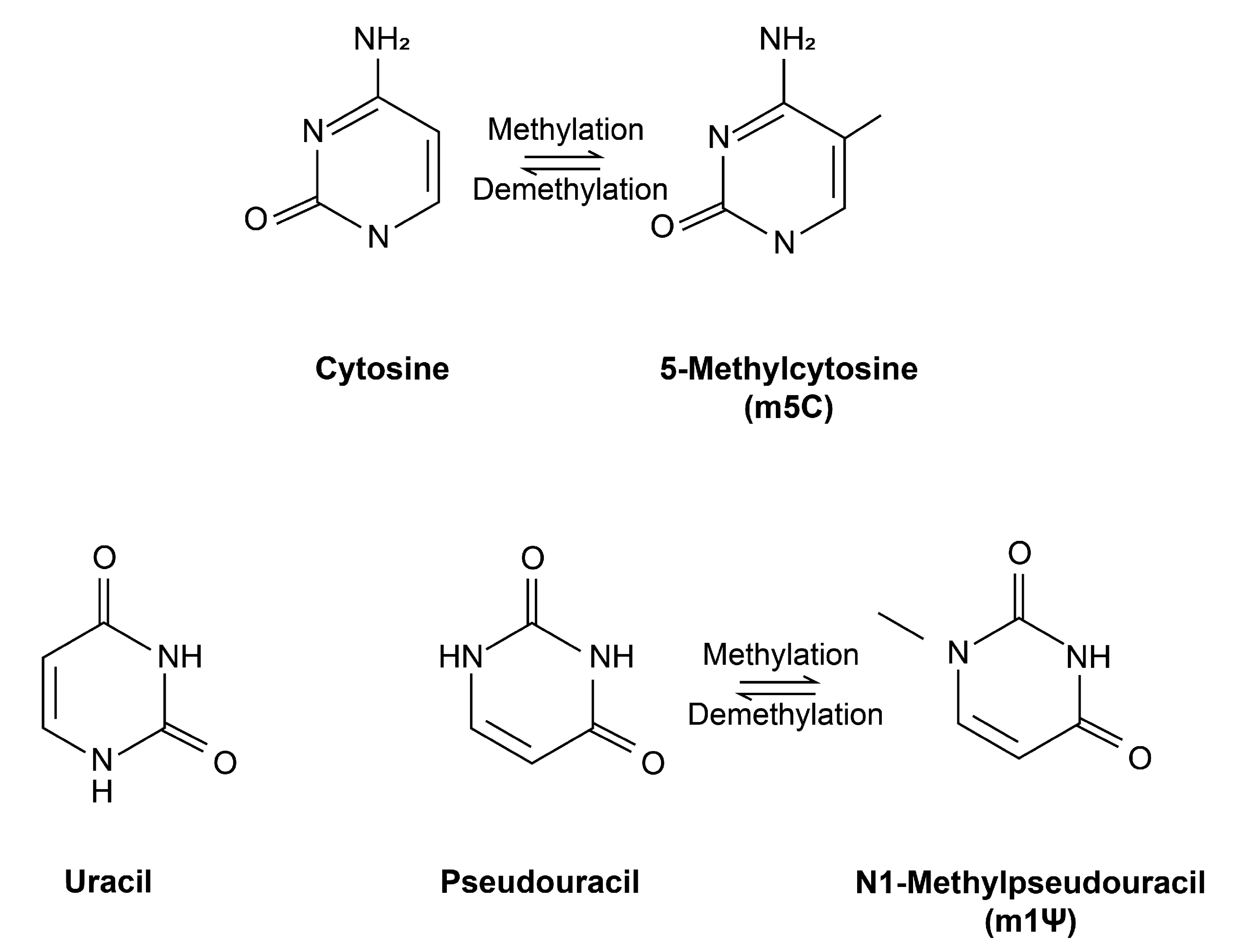
Figure 2. Structures of standard and modified nucleotides incorporated into mRNA therapeutics
Lipid nanoparticles
For an mRNA molecule to effectively pass into the cytoplasm of a cell to be translated, the mRNA molecule must be encapsulated by a lipid, as mRNA is too large and polar to freely pass through a lipid membrane. Rapid mixing of an ethanol phase containing lipids and an aqueous phase containing the mRNA molecules results in formation of small (~100 nm) lipid nanoparticles (LNPs) that have encapsulated the mRNA. These LNPs can fuse with the plasma membranes of cells and release mRNA into the cytoplasm.
An obstacle that LNPs must overcome for effective therapeutic delivery of mRNA is endosomal entrapment. Following fusion with the plasma membrane lipid nanoparticles are typically contained in the endosomal compartments of cells. If the mRNA remains in the endosome, it is unable to be translated and ultimately ends up degraded. Endosomal entrapment can be overcome by optimizing the pKa values of ionizable lipids and formulation of lipidic tails.
The exact composition of the lipids that compose the lipid nanoparticles can vary and be experimentally refined for different systems. The two main types of lipids that are used in most LNP formulations are cationic and ionizable. In addition, other types of lipids including cholesterols, phospholipids, and PEG can be added to the formulations to improve stability and biodistribution.
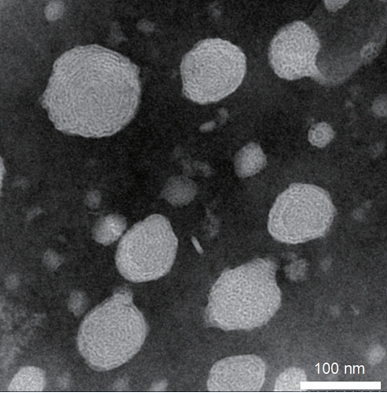
Figure 3. Transmission Electron Microscopy of lipid nanoparticles formulated at VectorBuilder
Conclusion
There are a variety of factors that influence the effectiveness of an mRNA therapeutic including mRNA design and lipid nanoparticle formulation. For each different mRNA therapeutic these factors need to be optimized dependent on the end goal of the therapeutic. mRNA therapeutics are currently being investigated in clinical trials to prevent and treat a wide range of disease including viral infections, cancer, metabolic disorders, and a variety of genetic diseases. The technology of mRNA therapeutics and lipid nanoparticles has been researched and worked on for the past 50+ years and has only recently emerged as a viable option for human therapeutics with the approval of the COVID-19 mRNA vaccines.
The future of mRNA therapeutics looks bright, and to learn how you can collaborate with VectorBuilder on your next mRNA project check out our mRNA Gene Delivery Solutions.
Sources
Qin, S., Tang, X., Chen, Y. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Sig Transduct Target Ther 7, 166 (2022). https://doi.org/10.1038/s41392-022-01007-w
Hou, X., Zaks, T., Langer, R. et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6, 1078–1094 (2021). https://doi.org/10.1038/s41578-021-00358-0



